Abstract
Objective
The Bologna motor and non-motor prospective study on parkinsonism at onset (BoProPark) was designed to prospectively characterize motor and non-motor features in patients with a progressive neurodegenerative disease starting with parkinsonism since early disease stage and to investigate their diagnostic and prognostic role in the differential diagnosis of Parkinson’s disease from atypical parkinsonisms. The aim of this paper is to describe the method and population of the BoProPark study.
Methods
Patients referred to our Department with parkinsonism within 3 years from motor onset were recruited. Secondary causes of parkinsonism were excluded. Each patient underwent a comprehensive evaluation of motor and non-motor symptoms, assessed by means of quantitative, objective instrumental tests in addition to scales and questionnaires. The evaluations were performed at enrolment (T0), after 16 months (T1) and after 5 years (T2). Diagnoses were made according to consensus criteria.
Results
We recruited 150 patients, with mean age 61.5 ± 9.8 years and mean disease duration 20 ± 9 months. H&Y stage was 1 in 47.3% and 2 in 46.7% of cases. Mean UPDRS-III was 17.7 ± 9.2. Fifty-four patients were on dopaminergic treatment with median levodopa equivalent daily dose (LEDD) of 200 mg.
Conclusions
We expect that the prospective nature of the BoProPark study as well as the comprehensive, instrumental evaluation of motor and non-motor symptoms in patients with parkinsonism will provide important new insights for both clinical practice and research. Our data could be used for comparison with other cohorts and shared with national and international collaborators to develop new innovative projects.
Similar content being viewed by others
Introduction
Parkinsonism is defined by the presence of a combination of bradykinesia, rigidity, tremor, and postural instability. It may be due to secondary causes (e.g., vascular encephalopathy, drugs’ side effects) or may occur in the context of neurodegenerative disorders, such as Parkinson’s disease (PD) or atypical parkinsonisms (APs). PD is the second most common neurodegenerative disease, carrying a high burden in terms of disability and healthcare cost worldwide [1]. APs are distinct entities, which at early disease stage could mimic PD [2], but during the disease course develop motor or non-motor features that exclude or are atypical for PD. Moreover, APs are characterized by a worse prognosis and different therapeutic needs compared to PD.
Nowadays, with the better understanding of the pathogenesis of these conditions and the aim of the development of disease-modifying therapies [3,4,5], the need for finding suitable clinical and instrumental markers to confirm diagnosis and track progression has become of crucial importance. Several prospective studies have been promoted for this purpose, focusing on frequency, severity, impact on diagnosis, and prognosis of motor as well as non-motor symptoms (e.g., autonomic failure, sleep disorders, cognitive impairment). However, most of these studies mainly assessed non-motor symptoms by means of questionnaires and scales that, while being comprehensive and standardized screening tools, do not allow objective quantification and only capture patient self-reported symptoms [6]. Moreover, the majority of these studies targeted patients with a single diagnosis, even though differentiating PD from APs at onset is still challenging [7].
The Bologna motor and non-motor prospective study on parkinsonism at onset (BoProPark) was designed to prospectively characterize motor and non-motor features, assessed by means of quantitative, objective instrumental tests in addition to scales and questionnaires, in patients with a progressive neurodegenerative disease starting with parkinsonism since early disease stage and to investigate their diagnostic and prognostic role in the differential diagnosis of these diseases.
The aim of this paper is to describe the method and population of the BoProPark study.
Materials and methods
The BoProPark is a prospective, observational, single-center study.
We enrolled all consecutive patients aged 18–80 years presenting with parkinsonism (tremor, bradykinesia, rigidity, postural instability) with a progressive course and within 3 years from motor onset that were referred from September 2007 up to November 2018 to the Movement Disorders Clinic of the Department of Biomedical and Neuromotor Sciences, University of Bologna. The neurodegenerative origin of the parkinsonism was confirmed in all patients by pathological single-photon emission computerized tomography for imaging the dopamine transporter (SPECT DaTSCAN). Secondary causes of parkinsonism were excluded before enrollment by means of appropriate investigations including brain magnetic resonance imaging (MRI). Other exclusion criteria to enter the study were concurrent clinically severe medical or psychiatric disease that could have interfered with study results.
The study was initially supported by the strategic research program “Bando ricerca finalizzata 2006” of the Italian Ministry of Health (reference number RFPS2006-7-336374) and subsequently continued as independent research and approved by the Ethics Committee of the Local Health Authority of Bologna (reference number 09070). The study was performed in accordance with the Declaration of Helsinki.
All participants gave their written informed consent to participate in the study.
BoProPark protocol
According to the BoProPark study, each patient underwent the same protocol (Table 1) at baseline (T0), after 16 months (T1) and 5 years (T2). The time window between T0 and T1 evaluations was chosen in order to get information on the early evolution of the disease, i.e., within the first 5 years, when correct clinical diagnosis is more challenging [29]. The second follow-up (T2) was set at reasonable time to collect data on an established clinical diagnosis, considering that PD and APs have different progression and prognosis. Moreover, because inclusion criteria requires that patients have parkinsonism within 3 years from motor onset, this time frame allowed us to evaluate most of the APs over their disease course, taking into account that survival in this cases is usually less than 10 years.
Drug naive patients at T0 started levodopa plus dopa-decarboxylase inhibitor (carbidopa or benserazide) treatment titrated in 1 month up to 200 + 50 mg/die.
The protocol included the following evaluations:
-
(a)
History taking: age, sex, occupation, family history, past medical history, drug history and concomitant medications, age at disease (parkinsonism) onset, disease duration, characteristics of onset and progression, symptoms at initial presentation, occurrence of motor and non-motor symptoms (history consistent with bradykinesia, rigidity, tremor, postural instability, other additional movement disorders such as dystonia or myoclonus and their body distribution, falls, dysphagia, dysarthria, dysphonia, higher mental functions, depression, sleep disturbances, autonomic symptoms including symptoms suggestive of orthostatic hypotension, urinary urgency, frequency, incontinence, incomplete bladder emptying, sexual dysfunction, sweating abnormalities, constipation, diarrhea, vision disturbances); severity, timing and latency from disease onset were recorded for each symptom and sign; dopaminergic treatment.
-
(b)
Body mass index.
-
(c)
Neurological examination.
-
(d)
Quantification of motor impairment and disease severity by means of Unified PD Rating Scale - part III (UPDRS-III) and Hoehn & Yahr (H&Y) stage [8, 9].
-
(e)
Patients treated with levodopa underwent quantification of motor response to levodopa through a subacute challenge test with a standard oral dose of levodopa plus carbidopa or benserazide (100 + 25 mg) based on a kinetic-dynamic approach [10]. Drug naïve patients at T0 underwent this examination within 6 months after the end of levodopa titration. Patient’s motor response was objectively assessed before and after standard intervals from drug dose with finger tapping performed on a computer-based system; simultaneous blood venous samples were withdrawn for measuring levodopa plasma concentration; dyskinesias, when present, were also rated at the same times as motor responses by the Clinical Dyskinesia Rating Scale [30]; blood pressure in supine position and after 3 min of standing was measured before and 1 h after drug administration to detect possible levodopa-induced or worsened orthostatic hypotension.
-
(f)
Evaluation of autonomic control of the cardiovascular system through cardiovascular reflex tests performed according to standardized procedures with continuous monitoring of beat-to-beat blood pressure, heart rate, oronasal airflow, abdominal breathing, and peripheral vasomotor tone and video: tilt test (65° for 10 min), Valsalva’s maneuver (forced expiratory pressure of 40 mmHg for 15 s), deep breathing test (6 breaths/min), cold face test (cold stimulus on forehead for 1 min), handgrip test (1/3 of maximal effort for 5 min) [11, 12]; blood pressure and heart rate changes from baseline in response to these maneuvers were used to calculate indices of sympathetic and parasympathetic activity and baroreflex integrity and to detect the presence of orthostatic hypotension.
-
(g)
Presence of symptoms of autonomic dysfunction through the questionnaire Scales for Outcomes in Parkinson’s Disease – Autonomic (SCOPA-Aut) [13].
-
(h)
Sleep study by means of whole-night video-polysomnography: electroencephalogram (C3-A2, O2-A1, Cz-A1), right and left electrooculogram, surface electromyogram from submental, intercostalis, right and left extensor carpi radialis and tibialis anterior muscles, tracheal microphone, oronasal airflow, thoracic and abdominal respirogram, electrocardiogram, oxyhemoglobin saturation by means of finger oximeter and synchronized video recording [14].
-
(i)
Sleep questionnaires and scales: PD sleep scale 1 [15], REM sleep behavior disorder (RBD) questionnaire [16], Bologna questionnaire on sleepiness-related symptoms [17], Restless Legs Syndrome criteria according to the International Classification of Sleep Disorders (ICSD-3) [18] and International Restless Legs Syndrome Study Group rating scale [19, 20], and Epworth sleepiness scale [21].
-
(j)
Evaluation of quality of life by means of 39-item PD questionnaire [22].
-
(k)
Cognitive and behavioral comprehensive assessment evaluating global cognition, verbal and visual memory, attention, executive and visuospatial function, language, depression, and anxiety. Neuropsychological evaluation included the following tests corrected for age, sex, and education according to Italian standardizations: Mini-Mental State Examination [23], Simple Copy Design Test [24], Selective Visual Attention Test (Stroop Test) [25], Phonemic and Semantic Verbal Fluency Test [26], and the Brief Mental Deterioration Battery, consisting of Rey’s Auditory Verbal Learning Test (immediate and delayed recall) [24], Visual Search Test (Barrage test) [24], Immediate Visual Memory Test [24], and Simple Verbal Analogies Test [31]. The Battery outcomes is a measure of global cognitive functioning, called Final Result [32, 33]. All patients also filled the 21-items Beck’s Depression Inventory [27] and the State-Trait Anxiety Inventory [28].
Demographic, clinical, and instrumental data were collected using a standard and anonymous form and entered in an ad hoc database. This database was specifically developed in Microsoft.net framework by SparkBio Srl (Bologna, Italy) for the purpose of collecting and storing data of this study in a comprehensive, standard, and safe way and making subsequent statistical analysis more straightforward. Moreover, this database allowed us to identify potential missing or erroneous information that were immediately pursued to guarantee the completeness and accuracy of the data. Importantly, the database was also implemented with a deterministic algorithm based on international criteria for PD [34], Parkinson’ disease with dementia (PDD) [35, 36], multiple system atrophy (MSA) [37], dementia with Lewy bodies (DLB) [38], progressive supranuclear palsy (PSP) [39], and corticobasal syndrome (CBS) [40] that checked the information and automatically provided a diagnosis that was recorded in the database itself and available for comparison and confirmation at future visits. In this regard, the database was designed as a questionnaire for all relevant diagnostic information needed to make a diagnosis according to the aforementioned criteria. Patients not fulfilling any diagnostic criteria were diagnosed as unspecified atypical parkinsonism (uAP). All diagnoses were independently confirmed by three neurologists expert in movement disorders who were blinded to the diagnosis provided by the database. Diagnoses made at baseline (T0) were revisited at T1 and T2 based on the results obtained from these follow-up evaluations.
Results of tests and questionnaires were recorded in ad hoc databases for statistical analysis.
Patients were also followed up at our department according to clinical practice and were regularly assessed for routine visits every 6–12 months as needed.
Statistical analysis
In the present article, we performed a descriptive analysis using IBM SPSS Statistics software (version 25). The distribution of continuous variables was confirmed by visual analysis of the Q-Q plots and the Shapiro Wilk test. Continuous variables were expressed using mean and standard deviation while categorical variables using frequencies and proportions.
Results
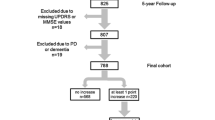
A total of 150 patients were recruited. Demographics and clinical characteristics at T0 are reported in Table 2. Mean age was 61.5 ± 9.8 years, and mean disease duration was 20 ± 9 months. H&Y stage was 1 in 47.3% and 2 in 46.7% of cases. Mean UPDRS III was 17.7 ± 9.2. Fifty-four patients were on dopaminergic treatment with median levodopa equivalent daily dose (LEDD) of 200 mg (36–700). The following diagnoses were made at T0: 108 PD, 4 MSA, 2 DLB, 3 PSP, 2 CBS, and 31 uAP (Fig. 1).
Study flowchart, PD = Parkinson’s disease; MSA = multiple system atrophy; DLB = dementia with Lewy bodies; PSP = progressive supranuclear palsy; CBS = corticobasal syndrome; uAP = unspecified atypical parkinsonism. (*) all uAP (according to data available). (**) 6 PD and 2 uAP (according to data available)
Discussion
The BoProPark study aims to describe and evaluate the diagnostic and prognostic role of motor and non-motor symptoms in a cohort of patients with a neurodegenerative disease starting with parkinsonism within 3 years from motor onset and prospectively followed up to reach a more robust clinical diagnosis. Motor and non-motor symptoms were evaluated by means of clinical and instrumental assessments according to standardized procedures. According to the results of clinical and instrumental assessments, diagnoses were revisited and confirmed at each evaluation (T0, T1, and T2) and at further follow-up.
In this paper, we describe study method and characteristics of patients at enrollment. Our cohort is representative of parkinsonian patients at disease onset referring to a tertiary Movement Disorder Center in Italy and Europe [41]. By further analyzing baseline and follow-up data, we expect to find specific clinical and instrumental markers for each domain assessed that can be helpful to the clinician in the diagnostic work-up and management of patient with parkinsonisms. Furthermore, our data could be used for comparison with other cohorts [6] and shared with national and international collaborators to develop new innovative projects. In addition, we could identify different patient populations with specific clinical characteristics that could be suitable for future studies assessing, for example, new and emerging MRI approaches like quantitative susceptibility mapping that might provide additional useful information for the early detection of PD and APs. We believe that our study will have important implications for clinical practice as well as in the research field.
Abbreviations
- PD:
-
Parkinson’s disease
- MSA:
-
Multiple system atrophy
- DLB:
-
Dementia with Lewy bodies
- PSP:
-
Progressive supranuclear palsy
- CBS:
-
Corticobasal syndrome
- uAP:
-
Unspecified atypical parkinsonism
- N :
-
Number of patients
- UPDRS-III:
-
Unified PD Rating Scale – part III
- LEDD:
-
Levodopa equivalent daily dose
References
Ray Dorsey E, Elbaz A, Nichols E et al (2018) Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 17:939–953. https://doi.org/10.1016/S1474-4422(18)30295-3
Ali K, Morris HR (2015) Parkinson’s disease: chameleons and mimics. Pract Neurol 15:14–25. https://doi.org/10.1136/practneurol-2014-000849
Elkouzi A, Vedam-Mai V, Eisinger RS, Okun MS (2019) Emerging therapies in Parkinson disease — repurposed drugs and new approaches. Nat Rev Neurol 15:204–223. https://doi.org/10.1038/s41582-019-0155-7
Boxer AL, Yu JT, Golbe LI, Litvan I, Lang AE, Höglinger GU (2017) Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol 16:552–563. https://doi.org/10.1016/S1474-4422(17)30157-6
Lopez-Cuina M, Foubert-Samier A, Tison F, Meissner WG (2018) Present and future of disease-modifying therapies in multiple system atrophy. Auton Neurosci Basic Clin 211:31–38. https://doi.org/10.1016/j.autneu.2017.12.008
Heinzel S, Lerche S, Maetzler W, Berg D (2017) Global, yet incomplete overview of cohort studies in Parkinson’s disease. J Park Dis 7:423–432. https://doi.org/10.3233/JPD-171100
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125:861–870. https://doi.org/10.1093/brain/awf080
Fahn S, Elton R, Members of the UPDRS Development Committee (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden C, Calne D, Goldstein M (eds) Recent developments in Parkinson’s disease, vol 153–63. Macmillan Health Care Information, Florham Park, pp 293–304
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression, and mortality. Neurology 17:427–442. https://doi.org/10.1212/WNL.17.5.427
Contin M, Riva R, Martinelli P, Albani F, Avoni P, Baruzzi A (2001) Levodopa therapy monitoring in patients with Parkinson disease: a kinetic-dynamic approach. Ther Drug Monit 23:621–629. https://doi.org/10.1097/00007691-200112000-00005
Corazza I, Barletta G, Guaraldi P, Cecere A, Calandra-Buonaura G, Altini E, Zannoli R, Cortelli P (2014) A new integrated instrumental approach to autonomic nervous system assessment. Comput Methods Prog Biomed 117:267–276. https://doi.org/10.1016/j.cmpb.2014.08.002
Ewing D (1978) Cardiovascular reflexes and autonomic neuropathy. Clin Sci Mol Med 55:321–327
Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ (2004) Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord 19:1306–1312. https://doi.org/10.1002/mds.20153
Vetrugno R, Provini F, Cortelli P, Plazzi G, Lotti EM, Pierangeli G, Canali C, Montagna P (2004) Sleep disorders in multiple system atrophy: a correlative video-polysomnographic study. Sleep Med 5:21–30. https://doi.org/10.1016/j.sleep.2003.07.002
Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, Pezzela FR, Forbes A, Högl B, Trenkwalder C (2002) The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry 73:629–635. https://doi.org/10.1136/jnnp.73.6.629
Scaglione C, Vignatelli L, Plazzi G, Marchese R, Negrotti A, Rizzo G, Lopane G, Bassein L, Maestri M, Bernardini S, Martinelli P, Abbruzzese G, Calzetti S, Bonuccelli U, Provini F, Coccagna G, Bologna, Genova, Parma and Pisa Universities group for the study of REM Sleep Behavior Disorder in Parkinson's Disease (2005) REM sleep behaviour disorder in Parkinson’s disease: a questionnaire-based study. Neurol Sci 25:316–321. https://doi.org/10.1007/s10072-004-0364-7
Rinaldi R, Vignatelli L, D’Alessandro R et al (2001) Validation of symptoms related to excessive daytime sleepiness. Neuroepidemiology 20:248–256
American Academy of Sleep Medicine (2014) International classification of sleep disorders, 3rd ed. Darien
Walters AS (1995) Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Mov Disord 10:634–642. https://doi.org/10.1002/mds.870100517
Walters AS, LeBrocq C, Dhar A et al (2003) Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 4:121–132. https://doi.org/10.1016/S1389-9457(02)00258-7
Vignatelli L, Plazzi G, Barbato A et al (2003) Italian version of the Epworth sleepiness scale: external validity. Neurol Sci 23:295–300. https://doi.org/10.1007/s100720300004
Peto V, Jenkinson C, Fitzpatrick R (1998) PDQ-39: a review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J Neurol 245:S10–S14. https://doi.org/10.1007/PL00007730
Measso G, Cavarzeran F, Zappalà G et al (1993) The mini-mental state examination: normative study of an Italian random sample. Dev Neuropsychol 9:77–85. https://doi.org/10.1080/87565649109540545
Carlesimo GA, Caltagirone C, Gainotti G et al (1996) The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol 36:378–384. https://doi.org/10.1159/000117297
Caffarra P, Vezzadini G, Dieci F et al (2002) Una versione abbreviata del test di Stroop: dati normativi nella popolazione italiana. Nuova Riv di Neurol 12:111–115
Novelli G, Papagno C, Capitani E et al (1986) Tre test clinici di ricerca e produzione lessicale. Taratura su sogetti normali. Arch Psicol Neurol Psichiatr 47:477–506
Beck AT, Ward CH, Mendelson M et al (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571. https://doi.org/10.1001/archpsyc.1961.01710120031004
Spielberger CD (1987) State-trait anxiety inventory. Anxiety 19:2009
Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E, Sabbagh MN, Sue LI, Jacobson SA, Belden CM, Dugger BN (2014) Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology 83:406–412. https://doi.org/10.1212/WNL.0000000000000641
Hagell P, Widner H (1999) Clinical rating of dyskinesias in Parkinson’s disease: use and reliability of a new rating scale. Mov Disord 14:448–455. https://doi.org/10.1002/1531-8257(199905)14:3<448::AID-MDS1010>3.0.CO;2-0
Gallassi R, Sambati L, Stanzani Maserati M, Poda R, Oppi F, de Matteis M, Marano G (2014) Simple verbal analogies test: normative data on a short task exploring abstract thinking. Aging Clin Exp Res 26:67–71. https://doi.org/10.1007/s40520-013-0180-0
Gallassi R, Lenzi P, Stracciari A, Lorusso S, Ciardulli C, Morreale A, Mussuto V (1986) Neuropsychological assessment of mental deterioration: purpose of a brief battery and a probabilistic definition of “normality” and “non-normality.”. Acta Psychiatr Scand 74:62–67. https://doi.org/10.1111/j.1600-0447.1986.tb06228.x
Gallassi R, Morreale A, Di Sarro R, Lorusso S (2002) Value of clinical data and neuropsychological measures in probable Alzheimer’s disease. Arch Gerontol Geriatr 34:123–134. https://doi.org/10.1016/S0167-4943(01)00204-7
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56:33–39. https://doi.org/10.1001/archneur.56.1.33
Emre M, Aarsland D, Brown R et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707
Dubois B, Burn D, Goetz C et al (2007) Diagnostic procedures for Parkinson’s disease dementia: recommendations from the Movement Disorder Society Task Force. Mov Disord 22:2314–2324. https://doi.org/10.1002/mds.21844
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676. https://doi.org/10.1212/01.wnl.0000324625.00404.15
McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M, Consortium on DLB (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 65:1863–1872
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47:1–9. https://doi.org/10.1212/WNL.47.1.1
Riley DE, Lang AE (2000) Clinical diagnostic criteria. In: Litvan I, Goetz CG, Lang AE (eds) Corticobasal degeneration and related disorders. Lippincott Williams & Wilkins, Philadelphia, pp 29–39
Baldereschi M, Di Carlo A, Rocca WA et al (2000) Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. Neurology 55:1358–1363. https://doi.org/10.1212/WNL.55.9.1358
Acknowledgments
This work is dedicated to professor Pasquale Montagna.
BoProPark Study Group collaborators
Giorgio Barletta1,2 (autonomic nervous system laboratory), Giuseppe Caltabiano1,2 (sleep laboratory), Annagrazia Cecere1,2 (autonomic nervous system laboratory), Roberto Gallassi (neuropsychology), Giulia Giannini1,2 (patients’ recruitment), Pietro Guaraldi1,3 (patients’ recruitment), Raffaele Lodi2,4 (neuroimaging), Giovanna Lopane1 (pharmacology), David Neil Manners4 (neuroimaging), Paolo Martinelli1 (patients’ recruitment), Filomena Miele1,2 (sleep laboratory), Francesco Mignani1,2 (sleep laboratory), Susan Mohamed1 (pharmacology), Stefania Nassetti1 (patients’ recruitment), Federico Oppi1 (neuropsychology), Piero Parchi1,2 (neuropathology), Giulia Pierangeli1,2 (autonomic nervous system laboratory), Roberto Poda1 (neuropsychology), Cesa Scaglione1 (patients’ recruitment), Laura Solieri1,2 (autonomic nervous system laboratory), Michelangelo Stanzani-Maserati1 (neuropsychology), Claudia Testa4 (neuroimaging)
1IRCCS Istituto delle Scienze Neurologiche di Bologna, Ospedale Bellaria, via Altura 3, 40138 Bologna, Italy
2Dipartimento di Scienze Biomediche e Neuromotorie, Università di Bologna, Bologna, Italy
3Neurology Outpatient Clinic, Department of Primary Care, Local Health Authority of Modena, Italy
4IRCCS Istituto delle Scienze Neurologiche di Bologna, Diagnostica Funzionale Neuroradiologica, Bologna, Italy
Funding
The study was supported by the strategic research program “Bando ricerca finalizzata 2006” of the Italian Ministry of Health (reference number RFPS2006-7-336374) and was co-financed with contributions from “5 x 1000” – support for neuroscience health research 2015.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethics Committee of the Local Health Authority 113 of Bologna (reference number 09070).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Calandra-Buonaura, G., Sambati, L., Baschieri, F. et al. The Bologna motor and non-motor prospective study on parkinsonism at onset (BoProPark): study design and population. Neurol Sci 41, 2531–2537 (2020). https://doi.org/10.1007/s10072-020-04305-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04305-9