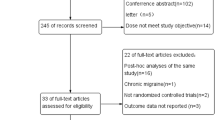
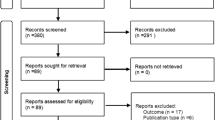
Abstract
Objective
This meta-analysis was performed to evaluate the efficacy and safety of monoclonal antibodies against calcitonin gene-related peptide (CGRP) for episodic migraine prevention.
Methods
MEDLINE, EMBASE, Web of Science, and the Cochrane Library were searched from inception to April 2018. Studies considered to be eligible were randomized controlled trials about efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine prevention.
Results
Eight randomized controlled trials involving 2292 patients were included. The outcomes of this meta-analysis presented that CGRP monoclonal antibodies for preventive treatment of episodic migraine significantly reduced the monthly migraine days from baseline [weighted mean difference (WMD) = − 1.52; 95%CI, − 1.92 to − 1.11; Z = 7.40; P < 0.001] and monthly acute migraine-specific medication consumption from baseline [WMD = − 1.45; 95%CI, − 2.17 to − 0.72; Z = 3.93; P < 0.001], as compared with placebo group. CGRP monoclonal antibodies for preventive treatment of episodic migraine significantly increased the ≥ 50% reduction from baseline in migraine days per month [RR = 1.54; 95%CI, 1.38 to1.71; Z = 7.88; P < 0.001]. The adverse events were similar between the CGRP monoclonal antibody group and placebo group (P = 0.998). The outcomes of subgroup analysis showed that erenumab, galcanezumab, and fremanezumab significantly reduced the monthly migraine days from baseline and increased the ≥ 50% reduction from baseline in migraine days per month. Both erenumab and fremanezumab significantly reduced from baseline.
Conclusions
Based on the results of this meta-analysis, CGRP monoclonal antibodies significantly reduced the monthly migraine days and acute migraine-specific medication. CGRP monoclonal antibodies were effective and safe for preventive treatment of episodic migraine.









Similar content being viewed by others
References
Headache Classification Committee of the International Headache Society (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33:629–808
Lipton RB, Manack Adams A, Buse DC, Fanning KM, Reed ML (2016) A comparison of the Chronic Migraine Epidemiology and Outcomes (CaMEO) study and American Migraine Prevalence and Prevention (AMPP) study: demographics and headache-related disability. Headache 56:1280–1289
Goadsby PJ, Sprenger T (2010) Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol 9:285–298
Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J (2002) CGRP may play a causative role in migraine. Cephalalgia 22:54–61
Ho TW, Edvinsson L, Goadsby PJ (2010) CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 6(10):573–582
Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94(4):1099–1142
Kaiser EA, Russo AF (2013) CGRP and migraine: could PACAP play a role too? Neuropeptides 47(6):451–461
Higgins JP, Green S Cochrane handbook for systematic reviews of interventions (version 51.0). [cited 2012 Jan 5]. Available at: http://www.cochrane-handbook.org.
Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, Sapra S, Picard H, Mikol DD, Lenz RA (2017) A controlled trial of erenumab for episodic migraine. N Engl J Med 377(22):2123–2132
Tepper S, Ashina M, Reuter U et al (2017) Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 16(6):425–434
Sun H, Dodick DW, Silberstein S et al (2016) Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 15(4):382–390
Dodick DW, Goadsby PJ, Silberstein SD et al (2014) Safety and efficacy of ALD403, an antibody to calciton in gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 13(9):1100–1107
Skljarevski V, Oakes TM, Zhang Q, Ferguson MB, Martinez J, Camporeale A, Johnson KW, Shan Q, Carter J, Schacht A, Goadsby PJ, Dodick DW (2017) Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol 75:187–193. https://doi.org/10.1001/jamaneurol.2017.3859.
Dodick DW, Goadsby PJ, Spierings ELH et al (2014) Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 13:885–892
Cohen JM, Dodick DW, Yang R, Newman LC, Li T, Aycardi E, Bigal ME (2017) Fremanezumab as add-on treatment for patients treated with other migraine preventive medicines. Headache 57(9):1375–1384
Bigal ME, Dodick DW, Rapoport AM et al (2015) Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 14(11):1091–1100
Hong P, Wu X, Liu Y (2017) Calcitonin gene-related peptide monoclonal antibody for preventive treatment of episodic migraine: a meta analysis. Clin Neurol Neurosurg 154:74–78
Khan S, Olesen A, Ashina M et al (2017) CGRP, target for preventive therapy in migraine and cluster headache: systematic review of clinical data. Cephalalgia 333102417741297.
Mitsikostas DD, Reuter U (2017) Calcitonin gene-related peptide moncoclonal antibodies for migraine prevention: comparisons across randomized controlled studies. Curr Opin Neurol 30(3):272–280
Bigal ME, Dodick DW, Krymchantowski AV (2016) TEV-48125 for the preventive treatment of chronic migraine: efficacy at early time points. Neurology 87(1):41–48
Giamberardino MA, Affaitati G, Martelletti P et al (2015) Impact of migraine on fibromyalgia symptoms. J Headache Pain 17(1):28
de Tommaso M, Sciruicchio V, Delussi M, Vecchio E, Goffredo M, Simeone M, Barbaro MGF (2017) Symptoms of central sensitization and comorbidity for juvenile fibromyalgia in childhood migraine: an observational study in a tertiary headache center. J Headache Pain 18(1):59
Cho SJ, Sohn JH, Bae JS, Chu MK (2017) Fibromyalgia among patients with chronic migraine and chronic tension-type headache: a multicenter prospective cross-sectional study. Headache 57(10):1583–1592
Ferrante E (2013) Modified Valsalva test differentiates primary from secondary cough headache. J Headache Pain 14(1):1–2
Adelborg K, Szépligeti SK, Holland-Bill L et al (2018) Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ 360:k96
Lipton RB, Reed ML, Kurth T, Fanning KM, Buse DC (2017) Framingham-based cardiovascular risk estimates among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache 57(10):1507–1521
Karp BI, Sinaii N, Nieman LK, Silberstein SD, Stratton P (2011) Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril 95(3):895–899
Kakisaka Y, Ohara T, Katayama S, Suzuki T, Hino-Fukuyo N, Uematsu M, Kure S (2013) Another case of lower back pain associated with migraine: the importance of specific questions. J Child Neurol 28(5):680
Yoon MS, Manack A, Schramm S, Fritsche G, Obermann M, Diener HC, Moebus S, Katsarava Z (2013) Chronic migraine and chronic tension-type headache are associated with concomitant low back pain: results of the German Headache Consortium study. Pain 154(3):484–492
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was not registered.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Yuhan Zhu is the first author and Yanyan Liu is the co-first author.
Rights and permissions
About this article
Cite this article
Zhu, Y., Liu, Y., Zhao, J. et al. The efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine: a meta-analysis. Neurol Sci 39, 2097–2106 (2018). https://doi.org/10.1007/s10072-018-3547-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3547-3