Abstract
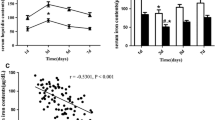
To study the changes in serum interleukin-11 (IL-11), tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF) expressions following hypertensive intracerebral hemorrhage (HICH), and explore their associations with disease severity and prognosis. Serum IL-11, TNF-α, and VEGF levels after 1, 3, 7, and 14 days after HICH were assayed using enzyme-linked immunosorbent assay (ELISA), and neurological deficit score (NDS) were recorded at admission and discharge for 99 HICH cases. Then 45 healthy controls were included and assayed for serum IL-11, TNF-α, and VEGF levels. Serum IL-11, TNF-α, and VEGF levels were higher in HICH patients than healthy controls (all P < 0.05). TNF-α was higher at the 3rd day following disease onset than other time points (all P < 0.05), while IL-11 and VEGF peaked at the 7th day and dropped below baseline values at the 14th day (all P < 0.05). Serum IL-11 was positively correlated with TNF-α (r = 0.70, P < 0.05) and VEGF (r = 0.72, P < 0.05). Serum TNF-α was positively correlated with VEGF (r = 0.46, P < 0.05). Serum IL-11, TNF-α, and VEGF were associated with disease severity in HICH patients. Patients with more severe disease tended to have higher NDS at admission, and higher IL-11, TNF-α, and VEGF during treatment were associated with higher NDS at discharge. Serum IL-11, TNF-α, and VEGF may involve in the pathophysiology of HICH, thus IL-11, TNF-α, and VEGF may be prognostic factors for post HICH neurologic damage.


Similar content being viewed by others
References
Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C (1994) Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke 25(2):333–337
Brott T, Thalinger K, Hertzberg V (1986) Hypertension as a risk factor for spontaneous intracerebral hemorrhage. Stroke 17(6):1078–1083
Tetri S, Juvela S, Saloheimo P, Pyhtinen J, Hillbom M (2009) Hypertension and diabetes as predictors of early death after spontaneous intracerebral hemorrhage. J Neurosurg 110(3):411–417
Biffi A, Anderson CD, Battey TW, Ayres AM, Greenberg SM, Viswanathan A, Rosand J (2015) Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA 314(9):904–912
Mirsen T (2010) Acute treatment of hypertensive intracerebral hemorrhage. Curr Treat Options Neurol 12(6):504–517
Yadav YR, Mukerji G, Shenoy R, Basoor A, Jain G, Nelson A (2007) Endoscopic management of hypertensive intraventricular haemorrhage with obstructive hydrocephalus. BMC Neurol 7(2):1–9
Abruzzo T, Patino M, Leach J, Rahme R, Geller J (2013) Cerebral vasoconstriction triggered by sympathomimetic drugs during intra-atrerial chemotherapy. Pediatr Neurol 48(2):139–142
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5(1):53–63
Mracsko E, Veltkamp R (2014) Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci 8(8):388
Ziai WC (2013) Hematology and inflammatory signaling of intracerebral hemorrhage. Stroke 44(6 Suppl 1):S74–S78
Bokhari FA, Shakoori TA, Butt A, Ghafoor F (2014) TNF-alpha: a risk factor for ischemic stroke. J Ayub Med Coll Abbottabad 26(2):111–114
Grivennikov SI (2013) IL-11: a prominent pro-tumorigenic member of the IL-6 family. Cancer Cell 24(2):145–147
Brynskov J, Foegh P, Pedersen G, Ellervik C, Kirkegaard T, Bingham A, Saermark T (2002) Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut 51(1):37–43
Ferrara N (1999) Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 56(55):794–814
Wang J, Dore S (2007) Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 27(5):894–908
Wang J (2010) Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol 92(4):463–477
Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M (2000) VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106(7):829–838
Fang HY, Ko WJ, Lin CY (2005) Plasma interleukin 11 levels correlate with outcome of spontaneous intracerebral hemorrhage. Surg Neurol 64(6):511–517 (discussion 517–518)
Beamer B, Hettrich C, Lane J (2010) Vascular endothelial growth factor: an essential component of angiogenesis and fracture healing. HSS J 6(1):85–94
Pn M (2014) World medical association publishes the revised Declaration of Helsinki. Natl Med J India 27(1):56
Schellinger PD, Bryan RN, Caplan LR, Detre JA, Edelman RR, Jaigobin C, Kidwell CS, Mohr JP, Sloan M, Sorensen AG, Warach S, Therapeutics, Technology Assessment Subcommittee of the American Academy of N (2010) Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 75(2):177–185
Neurology CSo (1996) Clinical standards for assessment of neurological deficit in stroke patients. Chin J Neurol 29(6):379–381
Zhang XM, Li XL, Tang SH, Liu QC (2006) Effect of head hypothermia on serum inflammatory cytokines levels in patients with hypertensive intracerebral hemorrhage. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 18(5):294–296
Joice SL, Mydeen F, Couraud PO et al (2009) Modulation of blood-brain barrier permeability by neutrophils: in vitro and in vivo studies. Brain Res 1298:13–23. doi:10.1016/j.brainres.2009.08.076
Suzuki K, Takano S, Nose T et al (1999) Increased concentration of vascular endothelial growth factor (VEGF) in chronic subdural hematoma. J Trauma 46(3):532–533
Silva Y, Leira R, Tejada J et al (2005) Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke 36(1):86–91
Zuo Y, Cheng G, Gao DK, Zhang X, Zhen HN, Zhang W, Xiao SC (2009) Gross-total hematoma removal of hypertensive basal ganglia hemorrhages: a long-term follow-up. J Neurol Sci 287(1–2):100–104
Wu J, Yang S, Xi G, Song S, Fu G, Keep RF, Hua Y (2008) Microglial activation and brain injury after intracerebral hemorrhage. Acta Neurochir Suppl 105(105):59–65
Saraswati S, Agarwal SS (2013) Strychnine inhibits inflammatory angiogenesis in mice via down regulation of VEGF, TNF-alpha and TGF-beta. Microvasc Res 87:7–13. doi:10.1016/j.mvr.2013.01.003
Honorati MC, Cattini L, Facchini A (2004) IL-17, IL-1beta and TNF-alpha stimulate VEGF production by dedifferentiated chondrocytes. Osteoarthr Cartil 12(9):683–691
Muir KW, Weir CJ, Murray GD, Povey C, Lees KR (1996) Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke 27(10):1817–1820
Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, Rosi S (2012) TNF-alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation 9:23. doi:10.1186/1742-2094-9-23
Stokes KY, Granger DN (2012) Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol 590(Pt 5):1023–1034
Hac-Wydro K (2013) Studies on beta-sitosterol and ceramide-induced alterations in the properties of cholesterol/sphingomyelin/ganglioside monolayers. Biochim Biophys Acta 1828(11):2460–2469
Kumar A, Loane DJ (2012) Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun 26(8):1191–1201
Acknowledgments
We would like to acknowledge the helpful comments on this paper received from our reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Rights and permissions
About this article
Cite this article
Yang, G., Shao, GF. Elevated serum IL-11, TNF α, and VEGF expressions contribute to the pathophysiology of hypertensive intracerebral hemorrhage (HICH). Neurol Sci 37, 1253–1259 (2016). https://doi.org/10.1007/s10072-016-2576-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-016-2576-z