Abstract
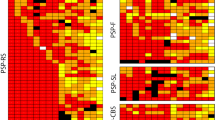
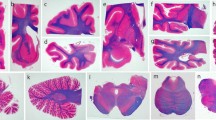
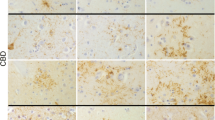
Progressive supranuclear palsy (PSP) is characterized neuropathologically by neuronal loss, gliosis, and the presence of tau-immunoreactive neuronal and glial cell inclusions affecting subcortical and some cortical regions. The objectives of this study were to determine (1) the spatial patterns of the tau-immunoreactive pathology, viz., neurofibrillary tangles (NFT), oligodendroglial inclusions (GI), tufted astrocytes (TA), and Alzheimer’s disease-type neuritic plaques (NP) in PSP and (2) to investigate the spatial correlations between the histological features. Post-mortem material of cortical and subcortical regions of eight PSP cases was studied. Spatial pattern analysis was applied to the NFT, GI, TA, NP, abnormally enlarged neurons (EN), surviving neurons, and glial cells. NFT, GI, and TA were distributed either at random or in regularly distributed clusters. The EN and NP were mainly randomly distributed. Clustering of NFT and EN was more frequent in the cortex and subcortical regions, respectively. Variations in NFT density were not spatially correlated with the densities of either GI or TA, but were positively correlated with the densities of EN and surviving neurons in some regions. (1) NFT were the most widespread tau-immunoreactive pathology in PSP being distributed randomly in subcortical regions and in regular clusters in cortical regions, (2) GI and TA were more localized and exhibited a regular pattern of clustering in subcortical regions, and (3) neuronal and glial cell pathologies were not spatially correlated.



Similar content being viewed by others
References
Steele JC, Richardson JC, Olszewski T (1964) Progressive supranuclear palsy. Arch Neurol 10:333–359
Schrag A, Ben-Shlomo Y, Quinn NP (1999) Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet 354:1771–1775
Nath U, Ben-Shlomo Y, Thomson RG et al (2001) The prevalence of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome) in the UK. Brain 124:1438–1449
Papapetropoulos S, Gonzalez J, Mash DC (2005) Natural history of progressive supranuclear palsy: a clinicopathologic study from a population of brain donors. Eur Neurol 54:1–9
Lantos PL (1994) The neuropathology of progressive supranuclear palsy. J Neural Transm (Suppl.) 42:137–152
Gomez-Haro C, Espert-Torbajada R, Gadea-Domenech M, Navarro-Humanes JF (1999) Progressive supranuclear palsy: neurological, neuropathological and neuropsychological aspects. Rev de Neurol 9:936–956
Armstrong RA, Lantos PL, Cairns NJ (2009) Hippocampal pathology in progressive supranuclear palsy (PSP): a quantitative study of 8 cases. Clin Neuropathol 28:46–53
Armstrong RA, Cairns NJ (2009) Laminar distribution of the pathological changes in frontal and temporal lobes in eight patients with progressive supranuclear palsy. Clin Neuropathol 28:350–357
Cordata NJ, Duggins AJ, Halliday GM, Morris JGL, Pantelis C (2005) Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain 128:1259–1266
Daniel SE, Bebruin VMS, Lees AJ (1995) The clinical and pathological spectrum of Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy)—a reappraisal. Brain 118:759–770
Tellez-Nagel I, Wisniewski HM (1973) Ultrastructure of neurofibrillary tangles in Steele–Richardson–Olszewski syndrome. Arch Neurol 29:324–327
Montpetit V, Clapin DF, Guberman M (1985) Substructure of 20 nm filaments of progressive supranuclear palsy. Acta Neuropathol 68:311–318
Dickson DW (1999) Neuropathological differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246:6–15
Dickson DW (2003) Neurodegeneration: the molecular pathology of dementia and movement disorders. International Society for Neuropathology (ISN) Press, Basel
Cairns NJ, Lee VM-Y, Trojanowski JQ (2004) The cytoskeleton in neurodegenerative disease. J Pathol 204:438–449
Mackenzie IRA, Hudson LP (1995) Achromatic neurons in the cortex of progressive supranuclear palsy. Acta Neuropathol 90:615–619
Yamada T, McGeer PL, McGeer EG (1992) Appearance of paired nucleated tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett 135:99–102
Ikeda K, Akiyama H, Kondo H et al (1995) Thorn-shaped astrocytes: possibly secondarily induced tau-positive glial fibrillary tangles. Acta Neuropathol 90:620–625
Komori T (1999) Tau positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol 9:663–679
Litvan I, Grimes DA, Lang AE, Jankovic J, McKee A, Verny M, Jellinger K, Chaudhuri KR, Pearce RKB (1999) Clinical features differentiating patients with postmortem confirmed progressive supranuclear palsy and corticobasal degeneration. J Neurol 246:1–5
Arima K, Nakamura M, Sunohara N et al (1999) Immunohistochemical and ultrastructural characterization of neuritic clusters around ghost tangles in the hippocampal formation in progressive supranuclear palsy. Acta Neuropathol 97:565–576
Armstrong RA, Cairns NJ, Lantos PL (2001) What does the study of spatial patterns tell us about the pathogenesis of neurodegenerative disorders? Neuropathology 21:1–12
De Lacoste M, White CL III (1993) The role of cortical connectivity in Alzheimer’s disease pathogenesis: a review and model system. Neurobiol Aging 14:1–16
Hiorns RW, Neal JW, Pearson RCA, Powell TPS (1991) Clustering of ipsilateral cortico-cortical projection neurons to area 7 in the rhesus monkey. Proc R Soc Lond 246:1–9
Armstrong RA, Lantos PL, Cairns NJ (2007) Spatial topography of the neurofibrillary tangles in cortical and subcortical regions in progressive supranuclear palsy. Parkinsonism Relat Disord 13:50–54
Litvan I, Agid Y, Calne D et al (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology 47:1–9
Litvan I, Hauw JJ, Bartko JJ et al (1996) Validity and reliability of the preliminary NINDS neuropathological criteria for progressive supranuclear palsy and related disorders. J Neuropath Exp Neurol 55:97–105
Hauw JJ, Daniel SE, Dickson D et al (1994) Preliminary NINDS neuropathologic criteria for Steele–Richardson–Olszewski syndrome (PSP). Neurology 44:2015–2019
Williams DR, Holton JL, Strand K, de Silva R, Lees AJ, Revesz T (2007) Differences in tau load are associated with different clinical phenotypes in progressive supranuclear palsy. Neuropathol Appl Neurobiol 33:256–257
Braak H, Braak E, Bohl J (1993) Staging of Alzheimer-related cortical destruction. Eur Neurol 33:403–408
Mattila P, Togo T, Dickson DW (2002) The subthalamic nucleus has neurofibrillary tangles in argyrophilic grain disease and in advanced Alzheimer’s disease. Neurosci Lett 320:81–85
Hattori M, Hashizume Y, Yoshida M, Iwasaki Y, Hishikawa N, Ueda R, Ojika K (2003) Distribution of astrocytic plaques in corticobasal degeneration brain and comparison with tuft shaped astrocytes in the progressive supranuclear palsy brain. Acta Neuropathol 106:143–149
Aoki K, Uchihara T, Nakamura A, Komori T, Arai N, Mizutani T (2003) Expression of apolipoprotein E in ballooned neurons: comparative immunohistochemical study in neurodegenerative disorders and infarctions. Acta Neuropathol 106:436–440
Fujino Y, Delucia MW, Davies P, Dickson DW (2004) Ballooned neurons in the limbic lobe are associated with Alzheimer-type pathology and lack diagnostic specificity. Neuropathol Appl Neurobiol 30:676–682
Minamu M, Mizutani T, Kawanishi R, Suzuki Y, Mori H (2003) Neuronal expression of alpha B crystalline in cerebral infarction. Acta Neuropathol 105:549–554
Armstrong RA (1993) The usefulness of spatial pattern analysis in understanding the pathogenesis of neurodegenerative disorders with special reference to plaque formation in Alzheimer’s disease. Neurodegeneration 2:73–80
Armstrong RA (2006) Methods of studying the planar distribution of objects in histological sections of brain tissue. J Microsc 221:153–158
Armstrong RA (2003) Measuring the degree of spatial correlation between histological features in thin sections of brain tissue. Neuropathology 23:245–253
Armstrong RA (1993) Is the clustering of neurofibrillary tangles in Alzheimer’s disease related to the cells of origin of specific cortico-cortical projections? Neurosci Lett 160:57–60
Armstrong RA, Cairns NJ, Lantos PL (1998) Clustering of Pick bodies in Pick’s disease. Neurosci Lett 242:81–84
Armstrong RA, Cairns NJ, Lantos PL (1999) Clustering of cerebral cortical lesions in patients with corticobasal degeneration. Neurosci Lett 268:5–8
Armstrong RA, Lantos PL, Cairns NJ (2007) Progressive supranuclear palsy (PSP): a quantitative study of the pathological changes in cortical and subcortical regions of eight cases. J Neural Transm 114:1569–1577
Mountcastle VB (1979) An organizing principle for cerebral function: the unit module and the distributed system. In: Schmitt FO and Worden FG (eds) The Neurosciences. 4th Study Program, pp 21–42
Brodal A (1981) Neurological Anatomy in relation to Clinical Medicine, 3rd edn. Oxford University Press, New York
Goedert M, Clavaguera F, Tolnay M (2010) The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci 33:317–325
Steiner JA, Angot E, Brunden P (2011) A deadly spread: cellular mechanisms of α-synuclein transfer. Cell Death Differ 18:1425–1433
Acknowledgments
We would like to thank the Department of Neuropathology, Institute of Psychiatry, King’s College London for supplying the cases of PSP and Heidi Barnes and Mavis Kibble for their excellent technical assistance. The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armstrong, R.A., Cairns, N.J. Spatial patterns of the tau pathology in progressive supranuclear palsy. Neurol Sci 34, 337–344 (2013). https://doi.org/10.1007/s10072-012-1006-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-012-1006-0