Abstract
Objective
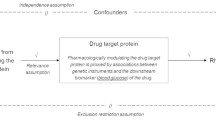
To evaluate the potential impact of consistent use of similar treatments over a long period; it is essential to investigate the potential correlation between genetic variations that influence the expression or function of pharmacological targets for reducing lipid levels and the risk of developing rheumatoid arthritis.
Methods
We used variants in the following genes to conduct Mendelian randomization analyses: HMGCR (encoding the target for statins), PCSK9 (encoding the target for PCSK9 inhibitors, such as evolocumab and alirocumab), and NPC1L1 (encoding the target for ezetimibe). Data from lipid genetics consortia (173,082 sample size) were used to weight variations according to their correlations with low-density lipoprotein cholesterol (LDL-C). In two large datasets (total n = 19,562 cases, 501,655 controls). We conducted a meta-analysis of Mendelian randomization estimates, weighted by LDL-C levels, on the regional differences in the risk of rheumatoid arthritis using data from two large databases.
Results
We approached SMR and IVW-MR analyses to examine the relationship between target gene expression (including HMGCR, PCSK9, and NPC1L1) and LDL-C levels mediated by these genes with RA. The IVW-MR analysis revealed no significant association between genetically predicted LDL-C concentration and the risk of RA (OR = 0.88, 95% CI = 0.59–1.29; OR = 0.91, 95% CI = 0.67–1.23; OR = 0.81, 95% CI = 0.49–1.36; all p > 0.05). Similarly, our findings from the SMR approach provided no evidence to suggest that gene expression of HMGCR, PCSK9, and NPC1L1 was associated with the risk of RA (OR = 0.91, 95% CI = 0.79–1.05, p = 0.207; OR = 0.96, 95% CI = 0.85–1.09, p = 0.493).
Conclusions
Our results do not provide evidence to support the hypothesis that reducing LDL-C levels with statins, alirocumab, or ezetimibe effectively prevents the risk of developing RA. However, our study provides valuable insights into the assessment of lipid-lowering agents in RA, which can enhance our understanding of the condition and assist in clinical practice by aiding in the determination and monitoring of RA status to clinical response.
Key Points • Common lipid-lowering drugs such as statins, alirocumab, or evolocumab may not effectively prevent the development of rheumatoid arthritis. • This study provides clues for further exploration of the role of lipid-lowering therapy in other high-risk diseases, contributing to a deeper understanding of the potential effects of lipid-lowering treatment. |



Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author/s.
References
Smith MH, Berman JR (2022) What Is rheumatoid arthritis? JAMA 327(12):1194. https://doi.org/10.1001/jama.2022.0786
Szekanecz Z, Koch AE, Tak PP (2011) Chemokine and chemokine receptor blockade in arthritis, a prototype of immune-mediated inflammatory diseases. Neth J Med 69(9):356–66
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423(6937):356–361. https://doi.org/10.1038/nature01661
Ngo ST, Steyn FJ, McCombe PA (2014) Gender differences in autoimmune disease. Front Neuroendocrinol 35(3):347–369. https://doi.org/10.1016/j.yfrne.2014.04.004
Turesson C, O’Fallon WM, Crowson CS et al (2003) Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis 62(8):722–727. https://doi.org/10.1136/ard.62.8.722
Nurmohamed MT, Dijkmans BA (2009) Dyslipidaemia, statins and rheumatoid arthritis. Ann Rheum Dis 68(4):453–455. https://doi.org/10.1136/ard.2008.104497
Heslinga M, Nurmohamed MT (2016) Cardiovascular Disease reduction in rheumatoid arthritis by statins: the final evidence? J Rheumatol 43(11):1950–1952. https://doi.org/10.3899/jrheum.161131
Koch CA, Krabbe S, Hehmke B (2018) Statins, metformin, proprotein-convertase-subtilisin-kexin type-9 (PCSK9) inhibitors and sex hormones: immunomodulatory properties? Rev Endocr Metab Disord 19(4):363–395. https://doi.org/10.1007/s11154-018-9478-8
Semb AG, Holme I, Kvien TK et al (2011) Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: an explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology 50(2):324–329. https://doi.org/10.1093/rheumatology/keq295. (Oxford)
Smith GD, Ebrahim S (2003) “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32(1):1–22. https://doi.org/10.1093/ije/dyg070
Yarmolinsky J, Wade KH, Richmond RC et al (2018) Causal inference in cancer epidemiology: what is the role of Mendelian randomization? Cancer Epidemiol Biomarkers Prev 27(9):995–1010. https://doi.org/10.1158/1055-9965.EPI-17-1177
Zhao YJ, Ong J, Goadsby PJ (2020) Emerging treatment options for migraine. Ann Acad Med Singap 49(4):226–235
Ference BA, Majeed F, Penumetcha R et al (2015) Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 x 2 factorial Mendelian randomization study. J Am Coll Cardiol 65(15):1552–1561. https://doi.org/10.1016/j.jacc.2015.02.020
Ference BA, Robinson JG, Brook RD et al (2016) Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 375(22):2144–2153. https://doi.org/10.1056/NEJMoa1604304
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–12. https://doi.org/10.1001/jama.283.15.2008
Plenge RM, Scolnick EM, Altshuler D (2013) Validating therapeutic targets through human genetics. Nat Rev Drug Discov 12(8):581–594. https://doi.org/10.1038/nrd4051
Li Z, Zhang B, Liu Q et al (2023) Genetic association of lipids and lipid-lowering drug target genes with non-alcoholic fatty liver disease. EBioMedicine 90:104543. https://doi.org/10.1016/j.ebiom.2023.104543
Li Z, Zhang B, Liu Q et al (2023) Genetic association of lipids and lipid-lowering drug target genes with non-alcoholic fatty liver disease. EBioMedicine 90:104543. https://doi.org/10.1016/j.ebiom.2023.104543
Williams DM, Finan C, Schmidt AF et al (2020) Lipid lowering and Alzheimer disease risk: a Mendelian randomization study. Ann Neurol 87(1):30–39. https://doi.org/10.1002/ana.25642
Willer CJ, Schmidt EM, Sengupta S et al (2013) Discovery and refinement of loci associated with lipid levels. Nat Genet 45(11):1274–1283. https://doi.org/10.1038/ng.2797
Okada Y, Wu D, Trynka G et al (2014) Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506(7488):376–381. https://doi.org/10.1038/nature12873
Ha E, Bae SC, Kim K (2021) Large-scale meta-analysis across East Asian and European populations updated genetic architecture and variant-driven biology of rheumatoid arthritis, identifying 11 novel susceptibility loci. Ann Rheum Dis 80(5):558–565. https://doi.org/10.1136/annrheumdis-2020-219065
Nikpay M, Goel A, Won HH et al (2015) A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 47(10):1121–1130. https://doi.org/10.1038/ng.3396
Zhu Z, Zhang F, Hu H et al (2016) Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet 48(5):481–487. https://doi.org/10.1038/ng.3538
Burgess S, Thompson SG (2011) Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 40(3):755–764. https://doi.org/10.1093/ije/dyr036
Zhu Z, Zhang F, Hu H et al (2016) Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet 48(5):481–487. https://doi.org/10.1038/ng.3538
Chauquet S, Zhu Z, O’Donovan MC et al (2021) Association of antihypertensive drug target genes with psychiatric disorders: a Mendelian randomization study. JAMA Psychiat 78(6):623–631. https://doi.org/10.1001/jamapsychiatry.2021.0005
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Burgess S, Thompson SG (2017) Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 32(5):377–389. https://doi.org/10.1007/s10654-017-0255-x
Verbanck M, Chen CY, Neale B et al (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50(5):693–698. https://doi.org/10.1038/s41588-018-0099-7
Nurmohamed MT, Dijkmans BA (2009) Dyslipidaemia, statins and rheumatoid arthritis. Ann Rheum Dis 68(4):453–455. https://doi.org/10.1136/ard.2008.104497
Wald NJ, Law MR (2003) A strategy to reduce cardiovascular disease by more than 80%. BMJ 326(7404):1419. https://doi.org/10.1136/bmj.326.7404.1419
Funk JL, Chen J, Downey KJ et al (2008) Bone protective effect of simvastatin in experimental arthritis. J Rheumatol 35(6):1083–1091
Abud-Mendoza C, de la Fuente H, Cuevas-Orta E et al (2003) Therapy with statins in patients with refractory rheumatic diseases: a preliminary study. Lupus 12(8):607–611. https://doi.org/10.1191/0961203303lu429oa
McCarey DW, McInnes IB, Madhok R et al (2004) Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 363(9426):2015–2021. https://doi.org/10.1016/S0140-6736(04)16449-0
McCarey DW, McInnes IB, Madhok R et al (2004) Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 363(9426):2015–2021. https://doi.org/10.1016/S0140-6736(04)16449-0
Navarro-Millan I, Charles-Schoeman C, Yang S et al (2013) Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum 65(6):1430–1438. https://doi.org/10.1002/art.37916
An J, Alemao E, Reynolds K et al (2016) Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol 43(11):1989–1996. https://doi.org/10.3899/jrheum.160110
DeBose-Boyd RA (2018) Significance and regulation of lipid metabolism. Semin Cell Dev Biol 81:97. https://doi.org/10.1016/j.semcdb.2017.12.003
Crowson CS, Rollefstad S, Ikdahl E et al (2018) Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 77(1):48–54. https://doi.org/10.1136/annrheumdis-2017-211735
Acknowledgements
We want to acknowledge the International Headache Genetics Consortium for providing summary data on migraine. We want to acknowledge the participants and investigators of the FinnGen study and the UK Biobank. We also want to acknowledge the participants and investigators of all other studies.
Funding
This work was supported by the Shanghai grassroots famous old Chinese medicine experts inheritance studio construction project (2020JCGZS-018) and Shanghai Xuhui District Medical Research Project (SHXH202221).
Author information
Authors and Affiliations
Contributions
LQ developed the protocol, participated in the literature search, extracted data, and drafted the manuscript; SL and KM were responsible for the analysis and interpretation of the data; J.Y was responsible for the critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval
Since we utilized publicly available GWAS summary data or published studies, ethical committee approval was not required for this manuscript.
Informed consent
Not applicable.
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiao, L., Lv, S., Meng, K. et al. Genetically proxied therapeutic inhibition of lipid-lowering drug targets and risk of rheumatoid arthritis disease: a Mendelian randomization study. Clin Rheumatol 43, 939–947 (2024). https://doi.org/10.1007/s10067-023-06837-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06837-9