Abstract
Background
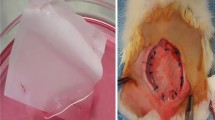
Decellularized porcine small intestinal submucosa (SIS) is a biological scaffold used surgically for tissue repair. Here, we demonstrate a model of SIS as a scaffold for human adipose-derived stem cells (ASCs) in vitro and apply it in vivo in a rat ventral hernia repair model.
Study design
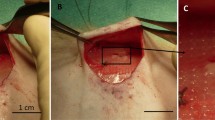
ASCs adherence was examined by confocal microscopy and proliferation rate was measured by growth curves. Multipotency of ASCs seeded onto SIS was tested using adipogenic, chondrogenic, and osteogenic induction media. For in vivo testing, midline abdominal musculofascial and peritoneal defects were created in Sprague–Dawley rats. Samples were evaluated for tensile strength, histopathology and immunohistochemistry.
Results
All test groups showed cell adherence and proliferation on SIS. Fibronectin-treated scaffolds retained more cells than those treated with vehicle alone (p < 0.05). Fresh stromal vascular fraction (SVF) pellets containing ASCs were injected onto the SIS scaffold and showed similar results to cultured ASCs. Maintenance of multipotency on SIS was confirmed by lineage-specific markers and dyes. Histopathology revealed neovascularization and cell influx to ASC-seeded SIS samples following animal implantation. ASC-seeded SIS appeared to offer a stronger repair than plain SIS, but these results were not statistically significant. Immunohistochemistry showed continued presence of cells of human origin in ASC-seeded repairs at 1 month postoperation.
Conclusion
Pretreatment of the scaffold with fibronectin offers a method to increase cell adhesion and delivery. ASCs maintain their immunophenotype and ability to differentiate while on SIS. Seeding freshly isolated SVF onto the scaffold demonstrated that minimally manipulated cells may be useful for perioperative surgical applications within the OR suite. We have shown that this model for a “living mesh” can be successfully used in abdominal wall reconstruction.





Similar content being viewed by others
Abbreviations
- SIS:
-
Small intestinal submucosa
- hASCs:
-
Human adipose-derived stem cells
- SVF:
-
Stromal vascular fraction
- FN:
-
Fibronectin
- CDT:
-
Cell doubling time
References
Millikan KW (2003) Incisional hernia repair. Surg Clin North Am 83(5):1223–1234
Leber GE et al (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133(4):378–382
Malangoni MA, Rosen MJ (2012) Hernias. In: Sabiston DC, Townsend CM (eds) Sabiston textbook of surgery: the biological basis of modern surgical practice. Elsevier Saunders, Philadelphia, pp 1114–1140
Slater NJ et al (2013) Biologic grafts for ventral hernia repair: a systematic review. Am J Surg 205(2):220–230
Lindroos B, Suuronen R, Miettinen S (2011) The potential of adipose stem cells in regenerative medicine. Stem Cell Rev 7(2):269–291
Iyyanki TS et al (2015) Adipose-derived stem-cell-seeded non-cross-linked porcine acellular dermal matrix increases cellular infiltration, vascular infiltration, and mechanical strength of ventral hernia repairs. Tissue Eng Part A 21(3–4):475–485
Smart NJ, Marshall M, Daniels IR (2012) Biological meshes: a review of their use in abdominal wall hernia repairs. Surgeon 10(3):159–171
Zhao Y et al (2012) Abdominal hernia repair with a decellularized dermal scaffold seeded with autologous bone marrow-derived mesenchymal stem cells. Artif Organs 36(3):247–255
McIlhenny SE et al (2010) Linear shear conditioning improves vascular graft retention of adipose-derived stem cells by upregulation of the alpha5beta1 integrin. Tissue Eng Part A 16(1):245–255
Kesler KA et al (1986) Enhanced strength of endothelial attachment on polyester elastomer and polytetrafluoroethylene graft surfaces with fibronectin substrate. J Vasc Surg 3(1):58–64
Ramalanjaona G et al (1986) The effect of fibronectin coating on endothelial cell kinetics in polytetrafluoroethylene grafts. J Vasc Surg 3(2):264–272
Zuk PA et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228
Kim SJ et al (2009) A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast (Ossron) injection to treat fractures. BMC Musculoskelet Disord 10:20
Tan BK et al (2007) Raised serum, adipocyte, and adipose tissue retinol-binding protein 4 in overweight women with polycystic ovary syndrome: effects of gonadal and adrenal steroids. J Clin Endocrinol Metab 92(7):2764–2772
Mo XT et al (2009) Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone 45(1):42–51
Steurer JA et al (2011) Chronic hernia repair in a rat model using small intestinal submucosa. J Invest Surg 24(5):227–235
Chen TT et al (2010) Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol 188(4):595–609
Chang HL et al (2010) Uterine leiomyomas exhibit fewer stem/progenitor cell characteristics when compared with corresponding normal myometrium. Reprod Sci 17(2):158–167
Hunt ME, Brown DR (2005) Mycoplasma alligatoris infection promotes CD95 (FasR) expression and apoptosis of primary cardiac fibroblasts. Clin Diagn Lab Immunol 12(12):1370–1377
Falco EE, Roth JS, Fisher JP (2008) EH Networks as a scaffold for skeletal muscle regeneration in abdominal wall hernia repair. J Surg Res 149(1):76–83
Wei RQ et al (2009) Grafts of porcine small intestinal submucosa with cultured autologous oral mucosal epithelial cells for esophageal repair in a canine model. Exp Biol Med (Maywood) 234(4):453–461
Qin HH, Dunn JC (2011) Small intestinal submucosa seeded with intestinal smooth muscle cells in a rodent jejunal interposition model. J Surg Res 171(1):e21–e26
Fann SA et al (2006) A model of tissue-engineered ventral hernia repair. J Invest Surg 19(3):193–205
Ayele T et al (2010) Tissue engineering approach to repair abdominal wall defects using cell-seeded bovine tunica vaginalis in a rabbit model. J Mater Sci Mater Med 21(5):1721–1730
Kern S et al (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5):1294–1301
Hunt GC, Singh P, Schwarzbauer JE (2012) Endogenous production of fibronectin is required for self-renewal of cultured mouse embryonic stem cells. Exp Cell Res 318(15):1820–1831
DeLise AM, Fischer L, Tuan RS (2000) Cellular interactions and signaling in cartilage development. Osteoarthr Cartil 8(5):309–334
Singh P, Schwarzbauer JE (2012) Fibronectin and stem cell differentiation—lessons from chondrogenesis. J Cell Sci 125(Pt 16):3703–3712
Wu W, Niklason L, Steinbacher DM (2013) The effect of age on human adipose-derived stem cells. Plast Reconstr Surg 131(1):27–37
Guadalajara H et al (2012) Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis 27(5):595–600
Acknowledgments
Support for this study was provided in part by NIH grant R01HL073980 (TNT) and DOD contract W81XW H08-2-00 (TNT). Biodesign was a kind gift of Cook Biotech (West Lafayette, IN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Klinger, A., Kawata, M., Villalobos, M. et al. Living scaffolds: surgical repair using scaffolds seeded with human adipose-derived stem cells. Hernia 20, 161–170 (2016). https://doi.org/10.1007/s10029-015-1415-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-015-1415-0