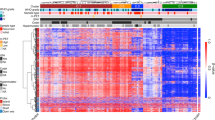
Abstract
Adult diffuse gliomas form a heterogeneous group of tumors of the central nervous system that vary greatly in histology and prognosis. A significant advance during the last decade has been the identification of a set of genetic lesions that correlate well with histology and clinical outcome in diffuse gliomas. Most characteristic driver mutations consist of isocitrate dehydrogenase 1 (IDH1) and IDH2, and H3 histone family member 3A, which are strongly associated with DNA and histone methylation patterns. A well-characterized DNA methylation aberration is on the O6-methylguanine-DNA methyltransferase promoter. This aberration is associated with an improved response to the DNA alkylating agent, temozolomide. Methylation alterations are used for classification or treatment decisions of diffuse gliomas. This supports the importance of considering epigenomic aberrations in the pathogenesis of gliomas. Recent DNA methylation analyses revealed a small group of IDH mutant diffuse gliomas exhibiting decreased DNA hypermethylation resulting in substantial unfavorable prognosis comparable to glioblastoma. Thus, DNA methylation patterns may become a new standard that replaces the conventional grading system based on histological diagnosis. In this review, we summarize recent developments regarding the contributions of methylation patterns to the pathogenesis of adult diffuse glioma, the interactions between methylation patterns and driver mutations, and potential epigenomic targeted therapies.


Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Aldape K, Simmons ML, Davis RL et al (2000) Discrepancies in diagnoses of neuroepithelial neoplasms: the San Francisco Bay Area Adult Glioma Study. Cancer 88:2342–2349
van den Bent MJ (2010) Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol 120:297–304
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773
Ichimura K (2012) Molecular pathogenesis of IDH mutations in gliomas. Brain Tumor Pathol 29:131–139
Cairncross JG, Ueki K, Zlatescu MC et al (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473–1479
Yamamichi A, Ohka F, Aoki K et al (2018) Immunohistochemical ATRX expression is not a surrogate for 1p19q codeletion. Brain Tumor Pathol 35:106–113
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Schwartzentruber J, Korshunov A, Liu XY et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231
Wu G, Broniscer A, McEachron TA et al (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251–253
Wu G, Diaz AK, Paugh BS et al (2014) The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46:444–450
Hermann A, Gowher H, Jeltsch A (2004) Biochemistry and biology of mammalian DNA methyltransferases. Cell Mol Life Sci 61:2571–2587
Quina AS, Buschbeck M, Di Croce L (2006) Chromatin structure and epigenetics. Biochem Pharmacol 72:1563–1569
Goll MG, Bestor TH (2005) Eukaryotic cytosine methyltransferases. Annu Rev Biochem 74:481–514
Huang Y, Rao A (2014) Connections between TET proteins and aberrant DNA modification in cancer. Trends Genet 30:464–474
Bird AP (1986) CpG-rich islands and the function of DNA methylation. Nature 321:209–213
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358:1148–1159
Esteller M (2007) Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet 16(Spec No 1):R50–R59
Berdasco M, Esteller M (2010) Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell 19:698–711
Costello JF, Berger MS, Huang HS et al (1996) Silencing of p16/CDKN2 expression in human gliomas by methylation and chromatin condensation. Cancer Res 56:2405–2410
Watanabe T, Yokoo H, Yokoo M et al (2001) Concurrent inactivation of RB1 and TP53 pathways in anaplastic oligodendrogliomas. J Neuropathol Exp Neurol 60:1181–1189
Nakamura M, Yonekawa Y, Kleihues P et al (2001) Promoter hypermethylation of the RB1 gene in glioblastomas. Lab Invest 81:77–82
Bello MJ, Rey JA (2006) The p53/Mdm2/p14(ARF) cell cycle control pathway genes may be inactivated by genetic and epigenetic mechanisms in gliomas. Cancer Genet Cytogen 164:172–173
Amatya VJ, Naumann U, Weller M et al (2005) TP53 promoter methylation in human gliomas. Acta neuropathologica 110:178–184
Lambiv WL, Vassallo I, Delorenzi M et al (2011) The Wnt inhibitory factor 1 (WIF1) is targeted in glioblastoma and has a tumor suppressing function potentially by induction of senescence. Neuro-Oncology 13:736–747
Gotze S, Wolter M, Reifenberger G et al (2010) Frequent promoter hypermethylation of Wnt pathway inhibitor genes in malignant astrocytic gliomas. Int J Cancer 126:2584–2593
Toyota M, Ahuja N, Ohe-Toyota M et al (1999) CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 96:8681–8686
Noushmehr H, Weisenberger DJ, Diefes K et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17:510–522
Turcan S, Rohle D, Goenka A et al (2012) IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483:479–483
Mardis ER, Ding L, Dooling DJ et al (2009) Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 361:1058–1066
Amary MF, Bacsi K, Maggiani F et al (2011) IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 224:334–343
Farshidfar F, Zheng S, Gingras MC et al (2017) Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep 19:2878–2880
Suzuki H, Aoki K, Chiba K et al (2015) Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet 47:458–468
Hartmann C, Meyer J, Balss J et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118:469–474
Dang L, White DW, Gross S et al (2010) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 465:966
Ward PS, Patel J, Wise DR et al (2010) The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17:225–234
Clark O, Yen K, Mellinghoff IK (2016) Molecular pathways: isocitrate dehydrogenase mutations in cancer. Clin Cancer Res 22:1837–1842
Bunse L, Pusch S, Bunse T et al (2018) Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med 24:1192–1203
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Juratli TA, Kirsch M, Geiger K et al (2012) The prognostic value of IDH mutations and MGMT promoter status in secondary high-grade gliomas. J Neurooncol 110:325–333
Stupp R, Hegi ME, Gorlia T et al (2014) Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 15:1100–1108
Baumert BG, Hegi ME, van den Bent MJ et al (2016) Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033–26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 17:1521–1532
Bady P, Delorenzi M, Hegi ME (2016) Sensitivity analysis of the MGMT-STP27 model and impact of genetic and epigenetic context to predict the MGMT methylation status in gliomas and other tumors. J Mol Diagn 18:350–361
Fernandez AF, Assenov Y, Martin-Subero JI et al (2012) A DNA methylation fingerprint of 1628 human samples. Genome Res 22:407–419
Moran S, Martinez-Cardus A, Sayols S et al (2016) Epigenetic profiling to classify cancer of unknown primary: a multicentre, retrospective analysis. Lancet Oncol 17:1386–1395
Sturm D, Orr BA, Toprak UH et al (2016) New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell 164:1060–1072
Sahm F, Schrimpf D, Stichel D et al (2017) DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 18:682–694
Capper D, Jones DTW, Sill M et al (2018) DNA methylation-based classification of central nervous system tumours. Nature 555:469–474
Ceccarelli M, Barthel FP, Malta TM et al (2016) Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164:550–563
Verhaak RG, Hoadley KA, Purdom E et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110
Brat DJ, Aldape K, Colman H et al (2018) cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol 136:805–810
Aoki K, Nakamura H, Suzuki H et al (2018) Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol 20:66–77
Cancer Genome Atlas Research N, Brat DJ, Verhaak RG et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498
Olar A, Wani KM, Alfaro-Munoz KD et al (2015) IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol 129:585–596
de Souza CF, Sabedot TS, Malta TM et al (2018) A distinct DNA methylation shift in a subset of glioma CpG island methylator phenotypes during tumor recurrence. Cell Rep 23:637–651
Mazor T, Pankov A, Johnson BE et al (2015) DNA methylation and somatic mutations converge on the cell cycle and define similar evolutionary histories in brain tumors. Cancer Cell 28:307–317
Bai H, Harmanci AS, Erson-Omay EZ et al (2016) Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat Genet 48:59–66
Yang X, Han H, De Carvalho DD et al (2014) Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell 26:577–590
Singer M, Kosti I, Pachter L et al (2015) A diverse epigenetic landscape at human exons with implication for expression. Nucleic Acids Res 43:3498–3508
Flavahan WA, Drier Y, Liau BB et al (2016) Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 529:110–114
Malta TM, de Souza CF, Sabedot TS et al (2018) Glioma CpG island methylator phenotype (G-CIMP): biological and clinical implications. Neuro Oncol 20:608–620
Jones PA, Issa JP, Baylin S (2016) Targeting the cancer epigenome for therapy. Nat Rev Genet 17:630–641
Lewis PW, Muller MM, Koletsky MS et al (2013) Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340:857–861
Mund C, Brueckner B, Lyko F (2006) Reactivation of epigenetically silenced genes by DNA methyltransferase inhibitors basic concepts and clinical applications. Epigenetics-Us 1:7–13
Issa JP (2005) Optimizing therapy with methylation inhibitors in myelodysplastic syndromes: dose, duration, and patient selection. Nat Clin Pract Oncol 2(Suppl 1):S24–S29
Kaminskas E, Farrell A, Abraham S et al (2005) Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clin Cancer Res 11:3604–3608
Tsai HC, Li H, Van Neste L et al (2012) Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell 21:430–446
Chiappinelli KB, Strissel PL, Desrichard A et al (2017) Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 169:361
Roulois D, Loo Yau H, Singhania R et al (2015) DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 162:961–973
Kantarjian HM, Roboz GJ, Kropf PL et al (2017) Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: phase 2 results from a multicentre, randomised, phase 1/2 trial. Lancet Oncol 18:1317–1326
Losman JA, Looper RE, Koivunen P et al (2013) R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 339:1621–1625
Stein EM (2015) IDH2 inhibition in AML: finally progress? Best Pract Res Cl Ha 28:112–115
Yen K, Travins J, Wang F et al (2017) AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov 7:478–493
Stein EM, DiNardo CD, Pollyea DA et al (2017) Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130:722–731
DiNardo CD, Stein EM, de Botton S et al (2018) Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. New Engl J Med 378:2386–2398
Rohle D, Popovici-Muller J, Palaskas N et al (2013) An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340:626–630
Tateishi K, Wakimoto H, Iafrate AJ et al (2015) Extreme vulnerability of IDH1 mutant cancers to NAD + depletion. Cancer Cell 28:773–784
Mazor T, Chesnelong C, Pankov A et al (2017) Clonal expansion and epigenetic reprogramming following deletion or amplification of mutant IDH1. Proc Natl Acad Sci USA 114:10743–10748
Mellinghoff IK, Touat M, Maher E et al (2017) Ag-120, a first-in-class mutant Idh1 inhibitor in patients with recurrent or progressive Idh1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro-Oncology 19:10–11
Acknowledgements
We would like to thank Editage (http://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aoki, K., Natsume, A. Overview of DNA methylation in adult diffuse gliomas. Brain Tumor Pathol 36, 84–91 (2019). https://doi.org/10.1007/s10014-019-00339-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-019-00339-w