Abstract
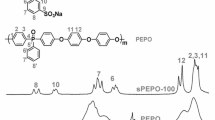
This study explored the concept of improving the properties of the cross-linked membrane using a 1.5-nm closed cage octaphenyl polyhedral silsesquioxane (POSS) form of nanosilica carrying the sulfonic acid group. POSS functioned with SO3H groups (SPOSS) at 0, 1, 2, and 5 wt% were cross-linked with water-soluble sulfonated polyphenylsulfone (SPPSU) polymer. The cross-linking between SPPSU and SPOSS was accomplished through the interchain condensation of sulfonic acid functionalities initiated by thermal curing treatment. In this study, a covalently cross-linked membrane was obtained under stepwise thermal curing from 80 to 180 °C. Upon curing at 180 °C, the SPPSU-SPOSS showed considerable improvement on the membrane proton conductivity under low and high RH (%) conditions compared with the pristine SPPSU membrane. The membrane proton conductivity shows similar patterns with the membrane water uptake as the presence of water greatly influences the cross-linked membrane. The proton conductivity of the SPPSU cross-linked with 1 wt% SPOSS that was conducted under low RH (%) and at elevated temperature exhibited about six times higher proton conductivity as compared with pristine SPPSU membrane. However, increasing the loading of SPOSS beyond 1 wt% significantly dropped the membrane water uptake and proton conductivity due to SPOSS aggregation, blocking the hydrophilic domains in the polymer matrix. The results indicated that the incorporation of SPOSS in the SPPSU membrane by curing at 180 °C exhibit improvement on membrane water management and proton conductivity as compared with the pristine SPPSU membrane.

Schematic diagram of proton transportation mechanisms in the SPPSU-SPOSS composite membranes








Similar content being viewed by others
References
Kirubakaran A, Jain S, Nema RK (2009) A review on fuel cell technologies and power electronic interface. Renew Sust Energ Rev 13(9):2430–2440
Miyake J, Taki R, Mochizuki T, Shimizu R, Akiyama R, Uchida M, Miyatake K (2017) Design of flexible polyphenylene proton conducting membrane for next generation fuel cells. Sci Adv 3:1–8
Muhmed SA, Nor NAM, Jaafar J, Ismail AF, Othman MHD, Rahman MA, Aziz F, Yusof N (2019) Emerging chitosan and cellulose green materials for ion exchange membrane fuel cell: a review. Energy Ecol Environ 2019:1–23
Tada M, Uruga T, Iwasawa Y (2015) Key factors affecting the performance and durability of cathode electrocatalysts in polymer electrolyte fuel cells characterized by in situ real time and spatially resolved XAFS techniques. Catal Lett 145(1):58–70
Kyu T, Nazir NA (2013) Supramolecules impregnated proton electrolyte membranes. Curr Opin Chem Eng 2(1):132–138
Park JW, Wycisk R, Pintauro PN, Yarlagadda V, Nguyen TV (2016) Electrospun nafion®/polyphenylsulfone composite membranes for regenerative hydrogen bromine fuel cells. Materials 9:1–15
Urena N, Perez-Prior MT, Rio CD, Varez A, Sanchez JY, Iojoiu C, Levenfeld B (2019) Multiblock copolymers of sulfonated PSU/PPSU poly (ether sulfone) s as solid electrolytes for proton exchange membrane fuel cells. Electrochim Acta 302:428–440
Kim YS, Pivovar BS (2010) Moving beyond mass-based parameters for conductivity analysis of sulfonated polymers. Annu Rev Chem Biomol Eng 1(1):123–148
Kim JD, Ghil LJ (2016) Annealing effect of highly sulfonated polyphenylsulfone polymer. Int J Hydrog Energy 41(27):11794–11800
Kamel MSA, Mohamed HFM, Abdel-Hamed MO, Abdel-Hady EE (2019) Characterization and evaluation of nafion HP jpJPs proton exchange membrane: transport properties, nanostructure, morphology, and cell performance. J Solid State Electrochem 23(9):2639–2656
Zhang J, Chen F, Ma X, Guan X, Chen D, Hickner MA (2015) Sulfonated polymers containing polyhedral oligomeric silsesquioxane (POSS) core for high performance proton exchange membranes. Int J Hydrog Energy 40(22):7135–7143
Zheng Y, Ash U, Pandey RP, Ozioko AG, Ponce-Gonzalez J, Handl M, Weissbach T, Varcoe JR, Holdcraft S, Liberatore MW, Hiesgen R, Dekel DR (2018) Water uptake study of anion exchange membranes. Macromolecules 51(9):3264–3278
Mazzapioda L, Panero S, Navarra MA (2019) Polymer electrolyte membranes based on nafion and a superacidic inorganic additive for fuel cell application. Polymers 11(5):914 (1-10)
Yen YC, Ye YS, Cheng CC, Lu CH, Tsai LD, Huang JM, Chang FC (2010) The effect of sulfonic acid groups within a polyhedral oligomeric silsesquioxane containing cross-linked proton exchange membrane. Polymer 51(1):84–91
Salarizadeh P, Javanbakht M, Pourmahdian S (2017) Enhancing the performance of speek polymer electrolyte membranes using functionalized TiO2 nanoparticles with proton hopping sites. RSC Adv 7(14):8303–8313
Shi S, Chen G, Wang Z, Chen X (2013) Mechanical properties of nafion 212 proton exchange membrane subjected to hygrothermal aging. J Power Sources 238:318–323
Kim K, Heo P, Hwang W, Baik JH, Sung YE, Lee JC (2018) Cross-linked sulfonated poly (arylene ether sulfone) containing a flexible and hydrophobic bishydroxy perfluoropolyether cross-linker for high-performance proton exchange membrane. Appl Mater Interfaces 10(26):21788–21793
Mokhtaruddin SR, Mohamad AB, Loh KS, Kadhum AAH (2016) Thermal properties and conductivity of nafion-zirconia composite membrane. Malays J Anal Sci 20(3):670–677
Awang N, Jaafar J, Ismail AF (2018) Thermal stability and water content study of void free electrospun speek/cloisite membrane for direct methanol fuel cell application. Polymers 10:1–16
Matsushita S, Kim JD (2018) Organic solvent-free preparation of electrolyte membranes with high proton conductivity using aromatic hydrocarbon polymers and small cross-linker molecules. Solid State Ionics 316:102–109
Wu Y, Li L, Feng S, Liu H (2013) Hybrid Nanocomposite based on novolac resin and octa (phenyl) polyhedral oligomeric silsesquioxanes (POSS): miscibility, specific interactions, and thermomechanical properties. Polym Bull 70(12):3261–3277
Lee CH, Park HB, Lee YM, Lee RD (2005) Importance of proton conductivity measurement in polymer electrolyte membrane for fuel cell application. Ind Eng Chem Res 44(20):7617–7626
Khabibullin A, Minteer SD, Zharov I (2014) The effect of sulfonic acid group content in pore-filled silica colloidal membranes on their proton conductivity and direct methanol fuel cell performance. J Mater Chem A 2(32):12761–12769
Kim SW, Choi SY, Rhee HW (2018) A novel sPEEK nanocomposite membrane with wee-controlled sPOSS aggregation in tunable nanochannels for past proton conduction. Nanoscales 10(38):18217–18227
Yameen B, Kaltbeitzel A, Glasser G, Langner A, Muller F, Gosele U, Knoll W, Azzaroni O (2010) Hybrid polymer-silicon proton conducting membranes via a pore-filing surface-initiated polymerization approach. ACS Appl Mater Interfaces 2(1):279–287
Sengupta S, Lyulin AV (2018) Molecular dynamics simulations of substrate hydrophilicity and confinement effects in capped nafion films. J Phys Chem B 122(22):6107–6119
Francia C, Ijeri VS, Specchia S, Spinelli P (2011) Estimation of hydrogen crossover through nafion membranes in PEMFCs. J Power Sources 196(4):1833–1839
O’Hayre R, Cha S, Colella W, Prinz FB (2009) Fuel cell fundamentals, Second edn. Wiley, New York
Niya SMR, Hoorfar M (2014) Process modelling of the ohmic loss in proton exchange membrane fuel cells. Electrochim Acta 120:193–203
Acknowledgments
The authors would like to express their gratitude to the Ministry of Higher Education (MOHE), Universiti Teknologi Malaysia (UTM) and Research Management Centre (RMC), UTM, for supporting the research management activities. The authors would also like to acknowledge support by the International Cooperative Graduate School (ICGS) Fellowship under the “Universiti Teknologi Malaysia-NIMS Cooperative Graduate School Program” to conduct research in the National Institute of Materials Science (NIMS), Tsukuba, Japan.
Funding
This work was supported by Ministry of Higher Education (MOHE) under project grant MRUN (R.J130000.7851.4L880) and Universiti Teknologi Malaysia (UTMPR:Q.J130000.2851.00L22, UTM-TDR:Q.J130000.3551.06G88). This work also was supported by the MEXT Program for the Development of Environmental Technology using Nanotechnology.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nor, N.A.M., Jaafar, J. & Kim, JD. Improved properties of sulfonated octaphenyl polyhedral silsequioxane cross-link with highly sulfonated polyphenylsulfone as proton exchange membrane. J Solid State Electrochem 24, 1185–1195 (2020). https://doi.org/10.1007/s10008-020-04594-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04594-2