Abstract
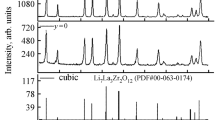
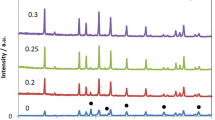
The limited electrochemical stability and the flammability of the liquid electrolytes presently used in Li-ion batteries stimulates the search for alternatives including ceramic solid electrolytes. Moreover, solid electrolytes also fulfil crucial functions in various large-scale energy storage systems, e.g. as anode-protecting membranes in aqueous Li-air batteries. Here, the processing of the solid electrolytes Li7La3Zr2O12 is studied for applications in Li-air batteries. Molten salt method (MSM) was adopted previously on synthesis of simple oxides; to the best of our knowledge, we report for the first time the adaptation of the MSM to prepare this class of solid electrolytes. As a model compound, we prepared the garnet-related Li6.75La3Zr1.75Ta0.25O12. It has been prepared by using stoichiometric amounts of La2O3, ZrCl4, and Ta2O5 in excess 0.88 M LiNO3:0.12 M LiCl molten salt. Subsequently, samples were heated to various temperatures in the range 600–900 °C for 6 h in air in a recrystallized alumina crucible and finally washed with distilled water to remove excess salts. The obtained Li6.75La3Zr1.75Ta0.25O12 electrolyte powder was characterized by X-ray diffraction, scanning electron microscopy, X-ray photoelectron spectroscopy, Raman, and impedance spectroscopy as well as surface area measurements. The cubic single phase was obtained for samples prepared at temperatures ≥700 °C. The effects of washing with water or aqueous LiOH solution on the structure and conductivity of the phases will be discussed.





Similar content being viewed by others
References
Knauth P (2009) Inorganic solid Li ion conductors: An overview. Solid state ionics 180: 911–916
Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A (2011) A lithium superionic conductor. Nat Mater 10: 682–686
Rao RP, Reddy MV, Adams S, Chowdari BVR (2012) Preparation and mobile ion transport studies of Ta and Nb doped Li6Zr2O7 Li-fast ion conductors. Materials science and engineering B-advanced functional solid-state materials 177:100–105
Chen MH, Yin XS, Reddy MV, Adams S (2015) All-solid-state MoS2/Li6PS5Br/In-Li batteries as a novel type of Li/S battery. J Mater Chem A 3:10698–10702
Safanama D, Damiano D, Rao RP, Adams S (2014) Lithium conducting solid electrolyte Li1+xAlxGe2 (-) (x)(PO4)(3) membrane for aqueous lithium air battery. Solid state ionics 262:211–215
Sun Y (2013) Lithium ion conducting membranes for lithium-air batteries. Nano Energy 2:801–816
Chen MH, Adams S (2015) All-solid-state lithium/sulfur batteries using lithium argyrodite electrolyte. J Solid State Electrochem 19:697–702
Murugan R, Thangadurai V, Weppner W (2007) Fast lithium ion conduction in garnet-type Li7La 3Zr2O12. Angewandte Chemie - International Edition 46:7778–7781
Rao RP, Gu WY, Sharma N, Peterson VK, Avdeev M, Adams S (2015) In Situ Neutron Diffraction Monitoring of Li7La3Zr2O12 Formation: Toward a Rational Synthesis of Garnet Solid Electrolyte. Chem Mater 27:2903–2910
Lee H, Yanilmaz M, Toprakci O, Fu K, Zhang X (2014) A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ Sci 7:3857–3886
Ohta S, Kobayashi T, Asaoka T (2011) High lithium ionic conductivity in the garnet-type oxide Li7-X La3(Zr2-X, NbX)O12 (X = 0-2) J Power Sources 196:3342–3345
Thangadurai V, Kaack H, Weppner WJF (2003) Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). J Am Ceram Soc 86:437–440
Adams S, Rao RP (2012) Ion transport and phase transition in Li7-xLa3(Zr2-xMx)O-12 (M = Ta5+, Nb5+, x=0, 0.25). J Mater Chem 22:1426–1434
Gu W, Ezbiri M, Rao RP, Avdeev M, Adams S (2015) Effects of penta- and trivalent dopants on structure and conductivity Of Li7La3Zr2O12. Solid State Ionics 274:100–105
Bottke P, Rettenwander D, Schmidt W, Amthauer G, Wilkening M (2015) Ion Dynamics in Solid Electrolytes: NMR Reveals the Elementary Steps of Li+ Hopping in the Garnet Li6.5La3Zr1.75Mo0.25O12. Chem Mater 27:6571–6582
Hubaud AA, Schroeder DJ, Key B, Ingram BJ, Dogan F, Vaughey JT (2013) Low temperature stabilization of cubic (Li7-xAl x/3)La3Zr2O12: Role of aluminum during formation. J Mater Chem A 1:8813–8818
Kumazaki S, Iriyama Y, Kim KH, Murugan R, Tanabe K, Yamamoto K, Hirayama T, Ogumi Z (2011) High lithium ion conductive Li7La3Zr 2O12 by inclusion of both Al and Si. Electrochem Commun 13:509–512
Rettenwander D, Langer J, Schmidt W, Arrer C, Harris KJ, Terskikh V, Goward GR, Wilkening M, Amthauer G (2015) Site occupation of Ga and Al in stabilized cubic Li7-3(x + y)GaxAlyLa3Zr2O12 garnets as deduced from 27Al and 71Ga MAS NMR at ultrahigh magnetic fields. Chem Mater 27:3135–3142
El-Shinawi H, Paterson GW, MacLaren DA, Cussen EJ, Corr SA (2017) Low-temperature densification of Al-doped Li7La3Zr2O12: a reliable and controllable synthesis of fast-ion conducting garnets. J Mater Chem A 5:319–329
Bernuy-Lopez C, Manalastas W, Lopez del Amo JM, Aguadero A, Aguesse F, Kilner JA (2014) Atmosphere Controlled Processing of Ga-Substituted Garnets for High Li-Ion Conductivity Ceramics. Chem Mater 26:3610–3617
Jalem R, Rushton MJD, Manalastas W Jr, Nakayama M, Kasuga T, Kilner JA, Grimes RW (2015) Effects of gallium doping in garnet-type Li7La3Zr2O12 solid electrolytes. Chem Mater 27:2821–2831
Song S, Yan B, Zheng F, Duong HM, Lu L (2014) Crystal structure, migration mechanism and electrochemical performance of Cr-stabilized garnet. Solid State Ionics 268:135–139
Hanc E, Zaja̧c W, Molenda J (2014) Synthesis procedure and effect of Nd, Ca and Nb doping on structure and electrical conductivity of Li7La3Zr2O 12 garnets. Solid State Ionics 262:617–621
Thangadurai V, Weppner W (2005) Li6ALa2Ta2O12 (A=Sr, Ba): Novel garnet-like oxides for fast lithium ion conduction. Adv Funct Mater 15:107–112
Thangadurai V, Narayanan S, Pinzaru D (2014) Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem Soc Rev 43:4714–4727
Li Y, Han J-T, Wang C-A, Vogel SC, Xie H, Xu M, Goodenough JB (2012) Ionic distribution and conductivity in lithium garnet Li7La3Zr2O12. J Power Sources 209:278–281
Shimonishi Y, Toda A, Zhang T, Hirano A, Imanishi N, Yamamoto O, Takeda Y (2011) Synthesis of garnet-type Li7 -XLa3Zr2O12 - 1/2x and its stability in aqueous solutions. Solid State Ionics 183:48–53
Jin Y, McGinn PJ (2011) Al-doped Li 7La 3Zr 2O 12 synthesized by a polymerized complex method. J Power Sources 196:8683–8687
Afyon S, Krumeich F, Rupp JLM (2015) A shortcut to garnet-type fast Li-ion conductors for all-solid state batteries. J Mate Chem A 3:18636–18648
Rosenkiewitz N, Schuhmacher J, Bockmeyer M, Deubener J (2015) Nitrogen-free sol-gel synthesis of Al-substituted cubic garnet Li7la3Zr2O12 (LLZO). J Power Sources 278:104–108
Xie H, Li Y, Goodenough JB (2012) Low-temperature synthesis of Li 7La 3Zr 2O 12 with cubic garnet-type structure. Mater Res Bull 47:1229–1232
Djenadic R, Botros M, Benel C, Clemens O, Indris S, Choudhary A, Bergfeldt T, Hahn H (2014) Nebulized spray pyrolysis of Al-doped Li7La3Zr 2O12 solid electrolyte for battery applications. Solid state ionics 263:49–56
Park JS, Cheng L, Zorba V, Mehta A, Cabana J, Chen G, Doeff MM, Richardson TJ, Park JH, Son JW, Hong WS (2015) Effects of crystallinity and impurities on the electrical conductivity of Li-La-Zr-O thin films. Thin solid films 576:55–60
Katsui H, Goto T (2015) Preparation of cubic and tetragonal Li7La3Zr2O12 film by metal organic chemical vapor deposition. Thin solid films 584:130–134
Yang T, Gordon ZD, Li Y, Chan CK (2015) Nanostructured Garnet-Type Solid Electrolytes for Lithium Batteries: Electrospinning Synthesis of Li7La3Zr2O12 Nanowires and Particle Size-Dependent Phase Transformation. J Phys Chem C 119:14947–14953
Tan KS, Reddy MV, Subba Rao GV, Chowdari BVR (2005) High-performance LiCoO2 by molten salt (LiNO3 : LiCl) synthesis for Li-ion batteries.J Power Sources 147:241–248
Zhao X, Reddy MV, Liu H, Ramakrishna S, Subba Rao GV, Chowdari BVR (2012) Nano LiMn2O4 with spherical morphology synthesized by a molten salt method as cathodes for lithium ion batteries. RSC Advances 2:7462–7469
Kim JH, Myung ST, Sun YK (2004) Molten salt synthesis of LiNi0.5Mn1.5O4 spinel for 5 V class cathode material of Li-ion secondary battery. Electrochim Acta 49:219–227
Reddy MV, Andreea LYT, Ling AY, Hwee JNC, Lin CA, Adams S, Loh KP, Mathe MK, Ozoemena KI, Chowdari BVR (2013) Effect of preparation temperature and cycling voltage range on molten salt method prepared SnO2. Electrochim Acta 106:143–148
Reddy MV, Sharma N, Adams S, Rao RP, Peterson VK, Chowdari BVR (2015) Evaluation of undoped and M-doped TiO2, where M = Sn, Fe, Ni/Nb, Zr, V, and Mn, for lithium-ion battery applications prepared by the molten-salt method. Rsc Advances 5:29535–29544
Afanasiev P, Geantet C (1998) Synthesis of solid materials in molten nitrates. Coord Chem Rev 178:1725–1752
Liu X, Fechler N, Antonietti M (2013) Salt melt synthesis of ceramics, semiconductors and carbon nanostructures. Chem Soc Rev 42:8237–8265
Larraz G, Orera A, Sanz J, Sobrados I, Diez-Gómez V, Sanjuán ML (2015) NMR study of Li distribution in Li7-xHxLa3Zr2O12 garnets. J Mater Chem A 3:5683–5691
Liu C, Rui K, Shen C, Badding ME, Zhang G, Wen Z (2015) Reversible ion exchange and structural stability of garnet-type Nb-doped Li7La3Zr2O12 in water for applications in lithium batteries. J Power Sources 282:286–293
Yow ZF, Oh YL, Gu W, Rao RP, Adams S (2016) Effect of Li+/H+ exchange in water treated Ta-doped Li7La3Zr2O12. Solid state ionics 292:122–129
Thompson T, Wolfenstine J, Allen JL, Johannes M, Huq A, David IN, Sakamoto J (2014) Tetragonal vs. cubic phase stability in Al - free Ta doped Li7La3Zr2O12 (LLZO). J Mater Chem A 2:13431–13436
Barsoukov E, Macdonald JR (2005) Impedance spectroscopy: theory, experiment, and applications
Langer F, Glenneberg J, Bardenhagen I, Kun R (2015) Synthesis of single phase cubic Al-substituted Li7La3Zr2O12 by solid state lithiation of mixed hydroxides. J Alloys Compd 645:64–69
Truong L, Thangadurai V (2011) Soft-chemistry of garnet-type Li 5+ xBa xLa 3-xNb 2O 12 (x = 0, 0.5, 1): Reversible H + ↔ Li + ion-exchange reaction and their X-ray, 7Li MAS NMR, IR, and AC impedance spectroscopy characterization. Chem Mater 23:3970–3977
Galven C, Fourquet JL, Crosnier-Lopez MP, Le Berre F (2011) Instability of the lithium garnet Li 7La 3Sn 2O 12: Li +/H + exchange and structural study. Chem Mater 23:1892–1900
Li Y, Han J-T, Vogel SC, Wang C-A (2015) The reaction of Li6.5La3Zr1.5Ta0.5O12 with water. Solid state ionics 269:57–61
Li Y, Han J-T, Wang C-A, Xie H, Goodenough JB (2012) Optimizing Li+ conductivity in a garnet framework. J Mater Chem 22:15357–15361
Nyman M, Alam TM, McIntyre SK, Bleier GC, Ingersoll D (2010) Alternative approach to increasing Li mobility in Li-La-Nb/Ta garnet electrolytes. Chem Mater 22:5401–5410
Galven C, Dittmer J, Suard E, Le Berre F, Crosnier-Lopez MP (2012) Instability of lithium garnets against moisture. Structural characterization and dynamics of Li 7-xH xLa 3Sn 2O 12 and Li 5-xH xLa 3Nb 2O 12. Chem Mater 24:3335–3345
Baral AK, Narayanan S, Ramezanipour F, Thangadurai V (2014) Evaluation of fundamental transport properties of Li-excess garnet-type Li5+2xLa3Ta2-xYxO12 (x = 0.25, 0.5 and 0.75) electrolytes using AC impedance and dielectric spectroscopy. PCCP 16:11356–11365
Acknowledgements
Authors wish to thank National Research Foundation, Prime Minister’s Office, Singapore for financial support under its Competitive Research Programme (CRP Award No. NRF-CRP 10-2012-6). Authors would like to thank Mr. Henche Kuan, Dept. of MSE, NUS for recording XPS data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contribution to Symposium A: “Advances in Energy Storage Systems: Lithium Batteries, Supercapacitors and Beyond”, during ICMAT 2015, June 28–July 3, Singapore
Rights and permissions
About this article
Cite this article
Reddy, M.V., Adams, S. Molten salt synthesis and characterization of fast ion conductor Li6.75La3Zr1.75Ta0.25O12 . J Solid State Electrochem 21, 2921–2928 (2017). https://doi.org/10.1007/s10008-017-3615-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3615-2