Abstract
Performance of dye-sensitized nano-crystalline TiO2 thin film-based photo-electrochemical solar cells (PECSCs) containing gel polymer electrolytes is largely governed by the nature of the cation in the electrolyte. Dependence of the photovoltaic performance in these quasi-solid state PECSCs on the alkaline cation size has already been investigated for single cation iodide salt-based electrolytes. The present study reports the ionic conductivity dependence on the nature of alkaline cations (counterion) in a gel polymer electrolyte based on binary iodides. Polyacrylonitrile-based gel polymer electrolyte series containing binary iodide salts is prepared using one of the alkaline iodides (LiI, NaI, KI, RbI, and CsI) and tetrapropylammonium iodide (Pr4NI). All the electrolytes based on binary salts have shown conductivity enhancement compared to their single cation counterparts. When combined with Pr4NI, each of the Li+, Na+, K+, Rb+, and Cs+ cation containing iodide salts incorporated in the gel electrolytes has shown a room temperature conductivity enhancement of 85.59, 12.03, 12.71, 20.77, and 15.36%, respectively. The conductivities of gel electrolytes containing binary iodide systems with Pr4NI and KI/RbI/CsI are higher and have shown values of 3.28, 3.43, and 3.23 mS cm−1, respectively at room temperature. The influence of the nature of counterions on the performance of quasi-solid state dye-sensitized solar cells is investigated by assembling two series of cells. All the binary cationic solar cells have shown more or less enhancements of open circuit voltage, short circuit current density, fill factor, and efficiency compared to their single cation counterparts. This work highlights the importance of employing binary cations (a large and a small) in electrolytes intended for quasi-solid state solar cells. The percentage of energy conversion efficiency enhancement shown for the PECSCs made with electrolytes containing Pr4NI along with Li+, Na+, K+, Rb+, and Cs+ iodides is 260.27, 133.65, 65.27, 25.32, and 8.36%, respectively. The highest efficiency of 4.93% is shown by the solar cell containing KI and Pr4NI. However, the highest enhancements of ionic conductivity as well as the energy conversion efficiency were exhibited by the PECSC made with Li+-containing binary cationic electrolyte.
Similar content being viewed by others
Introduction
Dye-sensitized, nano-crystalline TiO2 thin film-based photo-electrochemical solar cells (PECSCs) have attracted significant interest after the first report by O’Regan and Grätzel [1]. Within few years, the energy conversion efficiency of such cells has been improved to 12% [2–4]. Although, at present, these liquid electrolyte-based solar cells have reached an efficiency of about 14% [5], and quasi-solid state configuration have attracted much attention due to their better stability, non-flammability, negligible vapor pressure, and simpler cell preparation techniques [6–9]. Although the performance of dye-sensitized PECSCs is largely governed by the charge transport and the nature of the ions in the electrolyte [10–12], detailed studies of these aspects are lacking in the literature.
Despite the fact that gel polymer electrolytes have exhibited many advantages such as simple cell construction and higher physical and chemical stability, the energy conversion efficiencies of quasi-solid state PECSCs at present are low in particular due to the charge transport limitations in the bulk of the electrolyte as well as at the electrode/electrolyte interfaces. Several methods have been employed so far in order to increase the ionic conductivity in these quasi-solid state polymer electrolytes. The widely used methods incorporate suitably selected plasticizers and/or inorganic fillers to enhance the ionic conductivity [13–15]. It has been established that the nature and the concentration of ionic species in the electrolyte have a profound influence on the conductivity in these polymer electrolytes [16–18]. In particular, the size, shape, and charge density of ions have independent and profound influences on both the conductivity and the ionic strength in a quasi-solid (gel) polymer electrolytes [10, 17].
Many iodide salts have been investigated to be used in electrolytes for dye-sensitized PECSCs. LiI, NaI, KI, tetrapropylammonium iodide, and imidazolium iodide-based ionic liquids have been widely used in such electrolytes. The importance of iodide/triiodide transport in the electrolyte for the performance of such PECSCs is obvious, but the cation also has a vital role in the electron transfer dynamics in such cells [16, 19, 20].
Counterion effects of single cation iodide salts in quasi-solid PECSCs
Electrolytes based on single cation iodide salts have been tested in quasi-solid state dye-sensitized PECSCs in order to study the counterion effects on the cell performances. Shen et al. [16] have studied the effect of alkali-metal cations in polyethylene oxide (PEO)-based gel electrolytes suitable for quasi-solid state PECSCs. The results showed that the open circuit voltage (V oc) increased with the increased radius of the alkali metal cation and it has been correlated to the shift of electron Fermi level (E F) of TiO2. However, they could not observe a clear trend for the short circuit current density (J sc) variation, which in general correlates with the ion conduction in the electrolyte. Reported maximum short circuit current density was about 6 mA cm−1 [16]. In a previous report by our group, alkaline cations suitable for quasi-solid state PECSCs were investigated using a polyacrylonitrile (PAN) polymer-based electrolyte series [17]. The ionic conductivity in the electrolyte and solar cell parameters V oc, FF, and power efficiency of the cell series have increased with alkaline cation size. Thus, the highest efficiency of 3.48% for quasi-solid state PECSC series containing unary salts was given by the cell containing CsI [17].
Another study by our group was focused on finding the best salt candidate out of the quaternary ammonium (ammonium, tetraethylammonium, tetrapropylammonium, tetrabutylammonium, tetrapentylammonium, and tetrahexylammonium) iodide series for PECSCs [11]. Out of these salts incorporated into plasticized, PAN-based polymer electrolytes, the best solar cell efficiency was exhibited by tetrapropylammonium iodide-containing cell. The maximum efficiency of 4.30% and a J sc of 10.78 mA cm−2 were shown by the tetrapropylammonium iodide-based cell.
Counterion effects in binary iodide-based quasi-solid state PECSCs
In order to improve the solar cell performance by using mutually competing effects of small and large size cations in the electrolyte, mixed cation-iodide salt containing electrolyte systems has been investigated recently [20–22]. The general observation has been that larger cations improve the V oc in the cell, whereas the smaller cations improve the J sc. However, due to the complex nature of polymer electrolytes and due to many different competing effects that influence the mass transport in such electrolytes, a careful investigation is needed in order to understand the behavior of these binary salt systems.
The cation effect of binary iodide (tetrahexylammonium iodide and MgI2)-based quasi-solid dye-sensitized PECSCs was investigated using plasticized PAN-based electrolyte [21]. The electrolyte-containing salt weight ratio Hex4NI to MgI2 100:20 has shown the best solar cell performance highlighting the influence of the binary cations on the performance improvement of the cell. The highest energy conversion of efficiency exhibited by this system was 3.5% [21].
A PEC solar cell with the mixed iodide system, KI, and tetrapropylammonium iodide (Pr4NI) in a PAN-based gel electrolyte has shown the highest efficiency of 5.36% with the maximum J sc of 13.79 mA cm−2 reflecting an efficiency enhancement of about 20% with respect to the lower efficiency end member, Pr4NI and about 8% with respect to the higher efficiency end member, KI [20]. In another study, MgI2 and Pr4NI salt compositions in the gel electrolyte have been varied to optimize the efficiency of PECSCs [22]. The cell with binary cations having the MgI2:Pr4NI molar ratio 18.4:81.6 has shown the highest efficiency of 5.18% showing an efficiency enhancement of 25% compared to the higher efficiency end member with Pr4NI only. [22].
A study of PECSCs with an electrolyte having a binary mixture of alkaline cations of Cs+ and Li+ has also affirmed the advantages of having binary cations instead of a single cation in the electrolyte [23]. The electrolyte containing LiI:CsI with mass ratio 1:1 showed the highest solar cell performance with an energy conversion efficiency of 4.8% under the irradiation of one sun, highlighting an efficiency enhancement of about 23% for binary iodide system compared to single salt counterpart [23].
The mixed cation effect in dye-sensitized solar cells containing polyvinyl alcohol (PVA)-based gel polymer electrolytes has been investigated using KI and tetrabutylammonium iodide by Aziz et al. [24]. In that study, solar cell with 30% KI and 70% tetrabutylammonium iodide has given the highest efficiency of 5.8%. Efficiency enhancement by binary salt effect for dye-sensitized solar cells has also been demonstrated using poly (1-vinylpyrrolidone-covinyl acetate)-based gel polymer electrolytes with KI and Pr4NI as salts [25].
The present work was focused on finding the best binary iodide system to be used in PAN-based quasi-solid state photo-electrochemical solar cells without using any volatile solvents as plasticizers. Therefore, a series of PECSCs made with plasticized PAN-based gel electrolytes containing one of the five alkali iodide salts LiI, NaI, KI, RbI, or CsI along with Pr4NI were investigated. The focus was to investigate the dependence of solar cell performance on the nature of the alkaline cation coupled with the quaternary ammonium cation (Pr4N+) in a gel polymer electrolyte.
Experimental
Materials
The starting chemicals, tetrapropylammonium iodide (Pr4NI), alkaline metal iodides MI (M=Li, Na, K, Rb, Cs), polyacrylonitrile (PAN, Mw = 150,000), iodine (I2), ethylene carbonate (EC), and propylene carbonate (PC), all with purities greater than 98% were purchased from Aldrich. Prior to use, PAN was vacuum-dried for 24 h at 50 °C. Other materials were used as received. Conducting glass plates containing fluorine-doped tin oxide (FTO) with a sheet resistance of 7 Ω cm−2 and sensitizing dye ruthenium 535-bis TBA (tetra-butylammonium) (N719 dye) were purchased from Solaronix SA. Titania (TiO2) powders (purity ≥99.5) of two different particles sizes, P25 (particle size ∼21 nm) and P90 (particle size ∼14 nm), were purchased from Degussa.
Electrolyte sample preparation
Five different gel electrolyte samples containing binary iodides were prepared according to the stoichiometric relation (PAN)10(EC)25(PC)20(MI)1.2(Pr4NI)0.75(I2)0.12 in order to get a good gel and to keep the molar concentration of the electrolyte constant. In this formula, PAN represents one monomer of the polymer polyacrylonitrile. The weights of raw materials used to prepare the series of electrolytes are given in Table 1.
The relevant weights of EC, PC, and binary salts were mixed in a closed glass bottle and continuously stirred for about 2 h at 50 °C. Then, PAN was added to the mixture which was stirred further keeping it at 40 °C for about 1 h. Finally, iodine was added to the mixture and heated to ∼100 °C along with continuous stirring for a few more minutes until a homogeneous viscous solution was obtained. Then, the resultant electrolyte was allowed to cool down to room temperature and jellify, prior to be used in PECSCs and measurements.
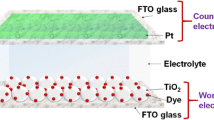
TiO2 photo-anode preparation
In order to prepare an efficient TiO2 photo-anode, two layers of TiO2 films were deposited on a conducting glass substrate. The first layer was spin-coated on a FTO substrate using a colloidal suspension of TiO2 P90 powder. The second layer was coated on the first layer with a colloidal suspension of TiO2 P25 powder using the doctor blade method.
The TiO2 photo-anode preparation has been described comprehensively in a previous work [26]. The dye adsorption to the double-layered TiO2 film overlaid on FTO glass plates was conducted by immersing the TiO2-coated FTO plates in a 0.5 mM ethanolic solution of ruthenium 535-bis TBA (N719 dye). The system was kept for 24 h at room temperature, in order to complete the dye adsorption. Then, the dye-adsorbed TiO2 electrodes were taken out from the dye solution and rinsed with acetone to remove the unbound TiO2 particles and loosely bound dye prior to assembling the solar cells.
In order to assemble the PECSCs, for each electrolyte composition, the gel electrolyte was casted onto the dye-adsorbed TiO2 electrode. Then, platinum (Pt)-coated conducting glass plate (counter electrode) was pressed on top of the TiO2 electrode to make the quasi-solid state dye-sensitized solar cells.
Measurements
Complex impedance measurements were taken on gel electrolyte samples by sandwiching the electrolyte between two stainless steel electrodes. The measurements were performed using a HP 4192A RF impedance analyzer in the 10 Hz–10 MHz frequency range. The temperature of the sample was decreased from 60 to 0 °C step-by-step for the measurements. The ionic conductivity of the samples was calculated by means of Nyquist plots.
Differential scanning calorimetry (DSC) thermograms for the electrolyte samples were obtained using a Mettler Toledo DSC 30 differential scanning calorimeter. Each sample was scanned between −140 and 100 °C at a rate of 10 °C min−1. The glass transition temperatures (T g) were extracted using the second heating cycle.
The PECSCs were illuminated by a LOT-Oriel GmbH solar simulator 1.5 AM, 1000 W m−2 (one sun) in order to obtain I-V characteristics using a computer-controlled eDAQ potentiostat and e-coder. The area of the cell exposed to light was 11 mm2, and the scan rate was 100 mV s−1.
Results and discussion
Electrolyte characterization
The ionic conductivities of two series of electrolytes one with single alkali cation iodide and the other with binary cation iodide were calculated using Nyquist plots. The ionic conductivity variation with the alkaline cations at room temperature (25 °C) is shown in Fig. 1 for these two series. For the single cation electrolytes, the higher conductivities were shown by the systems with larger cations, namely K+-, Rb+-, Cs+-containing electrolytes. Thus, the conductivity in Li+ and Na+ systems was poor. The room temperature conductivities of K+-, Rb+-, and Cs+-containing samples were about 2.9 mS cm−1. However, ionic conductivities in the binary cationic system have higher values than the corresponding values in the single cation system, as shown in Fig. 1. Out of the binary electrolyte series, systems with larger cations K+, Rb+, and Cs+ combined with Pr4N+ showed higher conductivities. The room temperature conductivities of binary iodide systems with K+, Rb+, and Cs+ were 3.28, 3.43, and 3.23 mS cm−1, respectively. The trend of higher conductivity exhibited for the binary cation system was common for all the temperatures within the measurement window (0–60 °C). The ionic conductivity enhancement obtained for the binary cation system could be attributed to the increase of ionic strength with added salt Pr4NI. There are so many effects imposed by the nature of cations for the conductivity in solid or gel polymer electrolytes and these effects have been discussed in detail for the single alkaline cation series-based electrolytes [17]. However, the dominant effect is the increase of ionic strength resulted in amalgamation of Pr4NI having a large cation. The exhibited room temperature conductivity enhancements with added Pr4NI relative to the corresponding single cation system were 85.59, 12.03, 12.71, 20.77, and 15.36% for Li+-, Na+-, K+-, Rb+-, and Cs+-containing electrolytes, respectively.
The conductivity isotherms of the electrolytes with binary salt are shown in Fig. 2. The conductivity increases with increasing temperature shown by all the samples is an expected behavior since the mobility and ionic strength increase with temperature [17]. As shown in Figs. 2, 3, the highest conductivity was shown by RbI-containing electrolyte for these series of electrolytes. However, KI- and CsI-containing samples have also given higher conductivities.
The higher conductivities shown by electrolytes containing larger cations can be explained as due to the possible reduction of local viscosity and improvement of polymer chain flexibility with increasing size of cations [17]. Larger cations facilitate ionic dissociation in addition, Li+, Na+, K+, Rb+, and Cs+ are hard Lewis acids and their strengths decrease with increasing ionic radius. Thus, the Lewis acid-base interactions with the hetero atoms in the polymer chain become weaker with increasing size of the cation. Therefore, ionic mobility could increase with increasing cation size.
On the other hand, the ion coordination number increases with the cation size. In addition, the viscous force is higher for that of larger cations. The slightly lower conductivity shown by the Cs+ (the largest cation in the series)-containing electrolyte compared to the Rb+- and K+-containing electrolytes could be attributed to these two effects.
The temperature dependence of the conductivity of electrolyte samples appears to follow the Vogel-Tammann-Fulcher (VTF) behavior as shown in Fig. 3 [23, 27, 28]. The conductivity data obtained for the two series of electrolytes were fitted to the VTF equation,
where, E a is a pseudo-activation energy, A is a pre-exponential factor and, k is the Boltzmann constant. The pseudo-activation energy E a is related to polymer segmental motion, which can be related to the mobility of charge carriers. T˳ is a reference temperature, the ideal glass transition temperature, usually associated with the temperature at which the free volume disappears. The glass transition temperature (T g) obtained using DSC measurements was used as T˳. The E a and pre-exponential factors obtained for different cations are shown in Fig. 4. The calculated E a values fluctuate around 31 and 33 meV. The pre-exponential factor A is proportional to the concentration of free charge carriers [27, 28]. The conductivity variation with temperature observed in this study correlates with A which is a measure of free charge carrier density.
Solar cell characterization
The open circuit photo-voltage (V oc) of the two series of quasi-solid state PECSCs with single and double cations is shown in Fig. 5. As revealed in a previous study, the V oc of the single cation system increases with increasing size of the cation [17]. All the binary cationic systems have shown efficiency enhancement with respect to their single cation counterparts. However, the V oc enhancement was very significant for the PECSC with the smallest cation Li+. A 39% enhancement in V oc was observed for LiI-containing PECSC (from 494 to 702 mV) when combined with Pr4NI. This significant enhancement of V oc with added Pr4NI, an iodide with relatively larger cation, can be attributed to the decrease of the recombination of electrons in the conduction band of TiO2 with I3 − in the electrolyte and to the shift of the resulting conduction band due to the cation adsorption by TiO2. A similar drop in V oc due to the large size cations, such as Pr4N+ in the electrolyte in mixed cation systems has been reported by several other groups [11, 17, 20, 22, 26].
The increase of V oc with the size of the counterion is already an established phenomenon for single cation systems [29]. However, the behavior of V oc in these quasi-solid state PECSCs with electrolyte containing binary iodides is not easy to understand. The increasing trend of V oc has diminished with increasing size of the counterion as seen from Fig. 5. The percentage of V oc enhancement in binary system compared to that of single cation counterparts was 42.1, 19.3, 9.2, 6.7, and 3.4%, respectively, for counterions Li+, Na+, K+, Rb+, and Cs+.
The ionic radius of the counterion in the added second salt (Pr4N+I), tetrapropylammonium ion (Pr4N+), is 4.6 Å; whereas, the ionic radius of Li+, Na+, K+, Rb+, and Cs+ is 0.76, 1.02, 1.38, 1.52, and 1.67 Å, respectively [17]. Interestingly, the binary system with the smallest and the largest cation, namely Li+ and Cs+, exhibited almost the same V oc of about 700 mV. However, K+ ion containing binary electrolyte showed the lowest V oc. This behavior might be due to the competing effects of binary cations to shift the conduction band positions of TiO2 films and the effects of back electron transfer [20, 23, 29]. Nevertheless, a very significant V oc enhancement was observed when Pr4NI is added to the electrolyte containing LiI.
The short circuit current density (J sc) variations of both the series of quasi-solid state PECSCs with the nature of the counterion (alkali cation) are shown in Fig. 6. The binary salt system with mixed cations has shown a higher J sc compared to that of the single cation system, highlighting the importance of employing a binary cationic (a large and a small) electrolytes in quasi-solid state PECSCs. The highest J sc of binary salt system, 11.5 mA cm−1, was shown by RbI-containing solar cell. KI-containing PECSC has also shown a J sc of 11.4 mA cm−1. In the single cation system, also the highest J sc was shown by the RbI-containing cell, whereas that in the KI-containing cell was the penultimate. Thus, it can be concluded that higher short circuit current density could be obtained by using K+ or Rb+ as the counterion in PECSCs with mixed cation iodide electrolytes.s
The trend of fill factor (FF) variation of the two series of quasi-solid state PECSCs is shown in Fig. 7. The FF increased from 43.0 to 61.6% with the cation size in the solar cells based on single cation (counterions) electrolyte. The FF was low in the PECSCs containing smaller cations in the electrolyte. However, these PECSCs exhibited a significant FF increase with the addition of the Pr4NI. For instance, in the Li+ cation-based system, the FF increased from 43.0 to 64.1% with the addition of Pr4NI.
Gradually increasing FFs were shown by the binary cationic PECSCs with small counterions such as Li+, Na+, and K+ contrary to the trend shown by the single cation salt-based solar cells. However, as shown in Fig 7, the addition of Pr4NI as the second iodide salt was not favorable for the FF in the quasi-solid state PECSCs containing larger alkaline cations such as Rb+ and Cs+. Out of all the series of cells, the highest FF of 66.0% was demonstrated by the quasi-solid state PECSC made with KI and Pr4NI binary iodide combination. The result suggests the suitability of the presence of KI to enhance the FF in the binary cationic electrolytes used in dye-sensitized PECSC.
The trend of solar cell efficiency variation of the single and binary cation-based two series of quasi-solid state PECSCs is shown in Fig. 8. The energy conversion efficiencies in the solar cells based on a single cation electrolyte increased with the increasing size of the cation. Therefore, the maximum efficiency in this series, 3.48%, was shown by the CsI-containing PECSC. Subsequently, the LiI-containing cell showed the lowest efficiency, 0.75%, out of the series solar cells. Therefore, a remarkable efficiency enhancement of 364% was observed due to the change of alkaline cation in the electrolyte. It can be inferred from the Figs. 6, 7, and 8 that the gradual efficiency enhancement observed in the single cation system with the increasing size of the cation was governed by the increase of V oc, FF, and J sc.
The binary cation salt system has shown higher efficiencies compared to the single cation system, highlighting the importance of employing a binary cationic (a large and small) electrolytes in quasi-solid state PECSCs. Although, the larger cation-containing cells exhibited higher efficiencies for single cation-based solar cells, the best efficiency of the binary cation system was shown by the KI-containing cell. The binary salt system with KI and Pr4NI showed the efficiency of 4.93%. The RbI-containing PECSC has also shown the penultimate efficiency of 4.34%.
Based on the above findings, it can be concluded that in the binary iodide salt-based electrolytes with Pr4NI as the iodide salt with a large cation, KI with a small cation appears to be the most suitable candidate out of the alkaline series studied here. This is an important observation since KI is widely abundant on earth, nontoxic, and easy to handle. Further, all the quasi-solid state solar cells based on gel polymer electrolytes having a single alkaline cation have shown efficiency enhancement due to the added tetrapropylammonium iodide. The percentage of energy conversion efficiency enhancements recorded with added Pr4NI was 260.27, 133.65, 65.27, 25.32, and 8.36% for Li+-, Na+-, K+-, Rb+-, and Cs+-containing solar cells, respectively. This indicates that the binary mixtures of salts are appropriate to enhance efficiency in quasi- solid state PECSCs.
Conclusions
Polyacrylonitrile (PAN)-based, single cationic, and binary cationic gel polymer electrolytes were prepared using tetrapropylammonium iodide as one salt and one of the alkaline iodides as the other salt. All the electrolytes based on binary salts have shown conductivity enhancement compared to those of single cation salt systems. The room temperature conductivities of binary iodide systems with K+, Rb+, and Cs+ were 3.28, 3.43, and 3.23 mS cm−1, respectively.
In addition, the influence of the nature of alkaline cations on the performance of quasi-solid state PECSCs was investigated by studying the effect of single and binary cation salt mixtures. All the solar cells made with binary cationic electrolytes have shown a significant enhancement of open circuit voltage, short circuit current density, fill factor, and efficiency in comparison with solar cells made with their single cation counterparts. Therefore, this work highlights the importance of employing a binary cationic (a large and a small) electrolyte for quasi-solid state PECSCs. Further, V oc enhancements of 42.1, 19.3, 9.2, 6.7, and 3.4%, respectively, were observed for PECSCs made with one of the Li+, Na+, K+, Rb+, and Cs+ cation salts together with Pr4NI. The highest efficiency of 4.93% was shown by KI- and Pr4NI-containing solar cells out of the two series of quasi-solid state solar cells studied in this work. The RbI- and Pr4NI-containing solar cell has also exhibited the penultimate efficiency of 4.34%.
One of the important observations made in this work is the reported increasing trend of J sc with cation size in contrast to the variation seen for liquid electrolyte-based PECSCs, emphasizing the difference in charge transport mechanisms in quasi-solid state electrolytes and liquid electrolytes.
In the binary iodide salt-based electrolytes with Pr4NI as the iodide salt with a large cation, KI with a small cation appears to be the most suitable candidate out of the alkaline series studied here. This observation is very important since KI is widely abundant on earth, nontoxic, and easy to handle. In addition, all the PECSCs based on gel polymer electrolytes having a single alkaline cation have shown a significant efficiency enhancement due to the added tetrapropylammonium iodide. The percentage of energy conversion efficiency enhancements observed with added Pr4NI was 260.27, 133.65, 65.27, 25.32, and 8.36% for Li+-, Na+-, K+-, Rb+-, and Cs+-containing PECSCs, respectively. This uncovers that the binary mixtures of salts are appropriate to enhance efficiency in quasi-solid state PECSCs.
References
Regan BO, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Han L, Koide N, Chiba Y, Islam A, Komiya R, Fuke N, Fukui A, Yamanaka R (2005) Improvement of efficiency of dye-sensitized solar cells by reduction of internal resistance. Appl Phys Lett 86:213501
Yella A, Lee HW, Tsao HN, Yi C, Chandiran AK, Nazeeruddin MK, Diau EWG, Yeh CY, Zakeeruddin SM, Grätzel M (2011) Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 334(6056):629–634
Chiba Y, Islam A, Watanabe Y, Komiya R, Koide N, Han L (2006) Dye-sensitized solar cells with conversion efficiency of 11.1%. J Appl Phys 45(25):L638–L640
Kakiage K, Aoyama Y, Yano T, Oya K, Fujisawa J, Hanaya M (2015) Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor, carboxy-anchor dyes. Chem Commun 51:15894–15897
Wang L, Zhang H, Wang C, Ma T (2013) Highly stable gel-state dye-sensitized solar cells based on high soluble polyvinyl acetate. ACS Sustain Chem Eng 1(2):205–208
Kang J, Li W, Wang X et al (2004) Gel polymer electrolytes based on a novel quaternary ammonium salt for dye-sensitized solar cells. J Appl Electrochem 34:301–304
Zhang X, Yang H, Xiong H-M, Li F-Y, Xia Y-Y (2006) A quasi-solid-state dye-sensitized solar cell based on the stable polymer-grafted nanoparticle composite electrolyte. J Power Sources 160:1451–1455
Lan Z, Wu J-H, Wang D-B, Hao S-C, Lin J-M, Huang Y-F (2006) Quasi-solid state dye-sensitized solar cells based on gel polymer electrolyte with poly (acrylonitrile-co-styrene)/NaI+ I 2. Sol Energy 80:1483
Suait MS, Rahman MYA, Ahmad A (2015) Review on polymer electrolyte in dye-sensitized solar cells (DSSCs). Sol Energy 115:452–470
Bandara TMWJ, Jayasundara WJMJSR, Dissanayake MAKL, Furlani M, Albinsson I, Mellander B-E (2013) Effect of cation size on the performance of dye sensitized nanocrystalline TiO2 solar cells based on quasi-solid state PAN electrolytes containing quaternary ammonium iodides. Electrochim Acta 109:609–616
Shi Y, Wang Y, Zhang M, Dong X (2011) Influences of cation charge density on the photovoltaic performance of dye-sensitized solar cells: lithium, sodium, potassium, dimethylimidazolium. Phys Chem Chem Phys 13:14590
Wu J, Lan Z, Lin J, Huang M, Huang Y, Fan L, Luo G (2015) Electrolytes in dye-sensitized solar cells. Chem Rev 115:2136–2173
Stephan AM (2006) Review on gel polymer electrolytes for lithium batteries. Eur Polym J 42:21–42
Stephan AM, Nahm KS (2006) Review on composite polymer electrolytes for lithium batteries. Polymer 47:5952–5964
Shen X, Xu W, Xu J, Liang G, Yang H, Yao M (2008) Quasi-solid-state dye-sensitized solar cells based on gel electrolytes containing different alkali metal iodide salts. Solid State Ionics 179:2027–2030
Bandara TMWJ, Fernando HDNS, Furlani M, Albinsson I, Dissanayake MAKL, Ratnasekera JL, Mellander B-E (2016) Effect of the alkaline cation size on the 18 conductivity in gel polymer electrolytes, their influence on photo electrochemical solar cells. Phys Chem Chem Phys 18:10873–10881
Sequeira C, Santos D (eds) (2010) Polymer electrolytes: fundamentals, applications, Elsevier
Watson DF, Meyer GJ (2004) Cation effects in nanocrystalline solar cells. Coord Chem Rev 248:1391–1406
Dissanayake MAKL, Thotawatthage CA, Senadeera GKR, Bandara TMWJ, Jayasundera WJMJSR, Mellander B-E (2012) Efficiency enhancement by mixed cation effect in dye-sensitized solar cells with PAN based gel polymer electrolyte. J Photochem Photobiol A Chem 246:29–35
Bandara TMWJ, Dissanayake MAKL, Jayasundara WJMJSR, Albinsson I, Mellander B-E (2012) Efficiency enhancement in dye sensitized solar cells using gel polymer electrolytes based on a tetrahexylammonium iodide, MgI2 binary iodide system. Phys Chem Chem Phys 14:8620
Dissanayake MAKL, Thotawatthage CA, Senadeera GKR, Bandara TMWJ, Jayasundara WJMJSR, Mellander B-E (2013) Efficiency enhancement in dye sensitized solar cells based on PAN gel electrolyte with Pr4NI + MgI2 binary iodide salt mixture. J Appl Electrochem 43:891
Bandara TMWJ, Jayasundara WJMJSR, Dissanayake MAKL, Fernando HDNS, Furlani M, Albinsson I, Mellander B-E (2014) Quasi solid state polymer electrolyte with binary iodide salts for photo-electrochemical solar cells. J Hydrogen Energy 39:2997–3004
Aziz MF, Buraidah MH, Careem MA, Arof AK (2015) PVA based gel polymer electrolytes with mixed iodide salts (K+I−, Bu4N+I−) for dye-sensitized solar cell application. Electrochim Acta 182(10):217–223
Ming NH, Ramesh S, Ramesh K (2016) The potential of incorporation of binary salts, ionic liquid in P (VP-co-VAc) gel polymer electrolyte in electrochemical, photovoltaic performances. Scientific Reports 6:27630. doi:10.1038/srep27630
Bandara TMWJ, Jayasundara WJMJSR, Fernado HDNS, Dissanayake MAKL, De Silva LAA, Fernando PSL, Furlani M, Mellander B-E (2014) J Appl Electrochem 44:917–926
Kuo CW, Huang CW, Chen BK, Li WB, Chen PR, Ho TH, Tseng CG, Wu TY (2013) Enhanced ionic conductivity in PAN-PEGME-LiClO4-PC composite polymer electrolytes. Int J Electrochem Sci 8:3834–3850
Ding L, Shi J, Yang C, Dong S (1997) Ionic conductivity of solid polymer electrolytes based on modified alternating maleic anhydride copolymer with oligo (oxyethylene) side chains. Polym J 29:410–416
Wang H, Peter LM (2012) Influence of electrolyte cations on electron transport, electron transfer in dye-sensitized solar cells. J Phys Chem C 116(19):10468–10475
Acknowledgements
The authors wish to acknowledge the research support provided by Chalmers University of Technology, Gothenburg, Sweden, Carl Tryggers Foundation for Scientific Research, Swedish Research Council, and National Research Council of Sri Lanka (grant 11-196).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bandara, T.M.W.J., Fernando, H.D.N.S., Furlani, M. et al. Dependence of solar cell performance on the nature of alkaline counterion in gel polymer electrolytes containing binary iodides. J Solid State Electrochem 21, 1571–1578 (2017). https://doi.org/10.1007/s10008-017-3518-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3518-2