Abstract
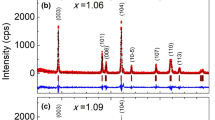
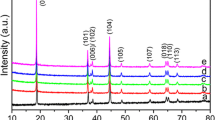
Li5SiN3 crystals are synthesized by direct reaction between Li3N and Si3N4 with the molar ratio Li3N/Si3N4 of 10:1. Reaction is performed at 1073 K for 1 h under a nitrogen atmosphere of 700 Torr. The lattice constant determined by the X-ray powder diffraction method is 4.718 Å. Four broad Raman peaks are observed at 196, 286, 580, and 750 cm−1. By analogy with LiMgN, the broad peak at 580 cm−1 with a half width of 140 cm−1 is attributed to homogenously random distribution of Li and Si atoms. The band gap of Li5SiN3 is found to be a direct gap of about 2.5 eV by optical absorption and photoacoustic spectroscopy methods. Comparison with the conventional cathode materials for lithium ion batteries, this gap value is close to d-d transition energy of Mn in LiMn2O4 (1.63 eV or 2.00 eV) and that of Co in LiCoO2 (2.1 eV), suggesting that Li5SiN3 is a possible cathode material. The 5 × 5 mm2-sized lithium secondary battery of Li5SiN3 cathode/propylene carbonate + LiClO4 electrolyte/Li anode structure shows a discharge capacity of 2.4 μAh cm−2 for a discharge current of 1.0 μA.






Similar content being viewed by others
References
Shokoohi FK, Tarascon JM, Wilkens BJ (1991) Appl Phys Lett 59:1260–1262
Wang B, Bates JB, Hart FX, Sales BC, Zuhr RA, Robertson JD (1996) J Electrochem Soc 143:3203–3213
Kushida K, Kuriyama K, Nozaki T (2002) Appl Phys Lett 81:5066–5068
Yamada Y, Nozaki T, Kuriyama K, Kushida K (2013) J Alloys and Compounds 551:44–47
Kushida K, Kuriyama K (2000) Appl Phys Lett 77:4154–4156
Kushida K, Kuriyama K (2001) Solid State Commun 118:615–618
Juza R, Hund F (1946) Naturwissenschaften 38:121–122
Yamane H, Kikkawa S, Koizumi M (1987) J Solid State Ionics 25:183–191
Tapia-Ruiz N, Segalés M, Gregory Duncan H (2013) Coord Chem Rev 257:1978–2014
Joint Committee for Powder Diffraction Standards (No. 40–1446)
Kuriyama K, Yamashita Y, Ishikawa T, Kushida K (2007) Phys Rev B75:233204–1-4
Kushida K, Kaneko Y, Kuriyama K (2004) Phys. Rev. B70:233303-1-4
Aoyama H, Kuwano S, Kuriyama K, Kushdia K (2013) J Alloys and Compounds 577:11–14
Juza R, Langer K, von Benda K (1968) Angew Chem Int Ed Engl 7:360–370
Kuriyama K, Kato T, Tanaka T (1994) Phys Rev B 49:4511–4513
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeuchi, Y., Yamashita, T., Kuriyama, K. et al. Synthesis and charge-discharge performance of Li5SiN3 as a cathode material of lithium secondary batteries. J Solid State Electrochem 20, 1885–1888 (2016). https://doi.org/10.1007/s10008-016-3131-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3131-9