Abstract
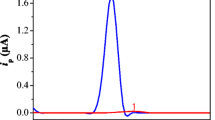
A sensitive electroanalytical method for the determination of anticancer drug etoposide (ETP) using adsorptive stripping differential pulse voltammetry (AdSDPV) at a multi-walled carbon nanotube-modified glassy carbon electrode (MWCNT-modified GCE) is presented. The surface morphology of modified electrode was characterized by scanning electron microscopy. The effects of accumulation time and potential, pH, scan rate, and amount of MWCNT suspension were investigated. The calibration curve was linear in the concentration range of 2.0 × 10−8–2.0 × 10−6 M with the detection limit of 5.4 × 10−9 M. The reproducibility of the peak current was found at 1.55 % (n = 5) RSD value in pH 6.0 Britton–Robinson buffer for the MWCNT-modified GCE. The method was then successfully utilized for the determination of ETP in pharmaceutical dosage form, and a recovery of 99.55 % was obtained. The possible oxidation mechanism of ETP was also discussed. The proposed electroanalytical method using MWCNT-modified GCE is the most sensitive method for the determination of ETP with lowest limit of detection in the previously published electrochemical methods.






Similar content being viewed by others
References
Gantchev TG, Hunting DJ (1998) The ortho-quinone metabolite of the anticancer drug etoposide (VP-16) is a potent inhibitor of the topoisomerase II/DNA cleavable complex. Mol Pharmacol 53:422–428
Brunton LL (ed) (2001) Goodman and Gilman's the pharmacological basis of therapeutics, 11th edn. McGraw Hill, New York
Sweetman SC (ed) (2007) Martindale: the complete drug reference, 25th edn. Pharmaceutical, London
Lakshmana Prabu S, Shahnawaz S, Kumar CD, Vasantharaju SG, Sivagurunathan N (2009) Spectrofluorimetric method for determination of etoposide in bulk and pharmaceutical dosage forms. Indian Drugs 46:58–60
Munawar Hayat M, Ashraf M, Rehman NU, Nasım FUH, Ahmad I, Rahman J, Saleem M, Malik MZ (2011) HPLC determination of etoposide in injectable dosage forms. J Chil Chem Soc 56:881–883
Cao YL, Du XL, Zhu Z, Fu Q (2011) HPLC assay for determination of etoposide in human plasma. Chin Pharm J 46:857–859
Demperio VL (2002) Determination of etoposide phosphate intermediates by gradient liquid chromatography using postcolumn derivatization with cuprammonium hydroxide. J Chromatogr A 952:283–287
Fahmy OT, Korany MA, Maher HM (2004) High performance liquid chromatographic determination of some co-administered anticancer drugs in pharmaceutical preparations and in spiked human plasma. J Pharm Biomed 34:1099–1107
Ashraf M, Hayat MM, Nasim FUH, Ahmad I, Saleem M, Rahman J (2012) Development and validation of RP-HPLC method for the simultaneous determination of etoposide and cisplatin and its application in quality control of injectable dosage forms. J Chem Soc Pak 34:321–325
Krogh-Madsen M, Hansen SH, Honore PH (2010) Simultaneous determination of cytosine arabinoside, daunorubicin and etoposide in human plasma. J Chromatogr B 878:1967–1972
Martin J, Camacho-Munoz D, Santos JL, Aparicio I, Alonso E (2011) Simultaneous determination of a selected group of cytostatic drugs in water using high-performance liquid chromatography-triple-quadrupole mass spectrometry. J Sep Sci 34:3166–3177
Tuerkab J, Kiffmeyera TK, Hadtsteinc C, Heinemannd A, Hahne M, Stuetzere H, Kussb HM, Eickmann U (2011) Development and validation of an LC–MS/MS procedure for environmental monitoring of eight cytostatic drugs in pharmacies. Intern J Environ Anal Chem 91:1178–1190
Ragozina NY, Pütz M, Heissler S, Faubel W, Pyell U (2004) Quantification of etoposide and etoposide phosphate in human plasma by micellar electrokinetic chromatography and near-field thermal lens detection. Anal Chem 76:3804–3809
Ozkan SA (2012) Electroanalytical methods in pharmaceutical analysis and their validation, 1st edn. HNB, New York
Holthuis JJM, Van Oort WJ, Römkens FMGM, Renema J, Zuman P (1985) Electrochemistry of podophyllotoxin derivatives: part I. Oxidation mechanism of etoposide (VP 16–213). J Electroanal Chem Interfacial Electrochem 184:317–329
Radi AE, Abd-Elghany N, Wahdan T (2007) Electrochemical study of the antineoplastic agent etoposide at carbon paste electrode and its determination in spiked human serum by differential pulse voltammetry. Chem Pharm Bull 55:1379–1382
Wang J (1998) Electroanalytical techniques in clinical chemistry and laboratory medicine. VCH, New York
Uslu B, Canbaz D (2010) Anodic voltammetry of zolmitriptan at boron-doped diamond electrode and its analytical applications. Die Pharm 65:245–250
Dogan-Topal B, Kul D, Ozkan SA, Uslu B (2011) Anodic behaviour of fulvestrant and its voltammetric determination in pharmaceuticals and human serum on highly boron-doped diamond electrode using differential pulse adsorptive stripping voltammetry. J Appl Electrochem 41:1253–1260
El Mhammedi MA, Bakasse M, Chtaini A (2007) Square-wave voltammetric determination of paraquat at carbon paste electrode modified with hydroxyapatite. Electroanal 19:1727–1733
Rezaei B, Damiri S (2010) Development of a voltammetric procedure for assay of thebaine at a multi-walled carbon nanotubes electrode: quantification and electrochemical studies. J Solid State Electr 14:1079–1088
Dogan-Topal B, Bozal-Palabıyık B, Uslu B, Ozkan SA (2013) Multi-walled carbon nanotube-modified glassy carbon electrode as a voltammetric nanosensor for the sensitive determination of anti-viral drug valganciclovir in pharmaceuticals. Sens Actuators B-Chem 177:841–847
Patil RH, Hegde RN, Nandibewoor ST (2011) Electro-oxidation and determination of antihistamine drug, cetirizine dihydrochloride at glassy carbon electrode modified with multi-walled carbon nanotubes. Colloid Surf B 83:133–138
Gasnier A, Pedano ML, Gutierrez F, Labbe P, Rivas GA, Rubianes MD (2012) Glassy carbon electrode modified with a dispersion of multi-wall carbon nanotubes in dopamine-functionalized polyethylenimine: characterization and analytical applications for nicotinamide adenine dinucleotide quantification. Electrochim Acta 71:73–81
Dirk M, Guldi NM (2010) Carbon nanotubes and related structures. Wiley, New York
Gosser DK (1993) Cyclic voltammetry: simulation and analysis of reaction mechanism. VCH, New York
Kachoosangi RT, Wildgoose GG, Compton RG (2008) Sensitive adsorptive stripping voltammetric determination of paracetamol at multiwalled carbon nanotube-modified basal plane pyrolytic graphite electrode. Anal Chim Acta 618:54–60
Grimshaw J (2000) Electrochemical reactions and mechanism in organic chemistry. Elsevier, New York
Bozkaya P, Dogan B, Suzen S, Nebioglu D, Özkan SA (2006) Determination and investigation of electrochemical behaviour of 2-phenylindole derivatives: discussion on possible mechanistic pathways. Can J Anal Sci Spectrosc 51:125–139
Riley CM, Rosanske TW (eds) (1996) Development and validation of analytical methods. Elsevier, New York
Ermer J, Miller McB JH (eds) (2005) Method validation in pharmaceutical analysis. Wiley, Weinheim
Swartz ME, Krull IS (1997) Analytical method development and validation. Marcel Dekker, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bozal-Palabiyik, B., Dogan-Topal, B., Uslu, B. et al. Sensitive voltammetric assay of etoposide using modified glassy carbon electrode with a dispersion of multi-walled carbon nanotube. J Solid State Electrochem 17, 2815–2822 (2013). https://doi.org/10.1007/s10008-013-2184-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2184-2