Abstract
Context
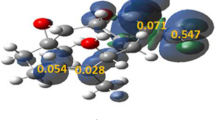
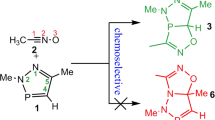
[3+2] cycloaddition processes between isoalantolactone (ISALL) and diazocyclopropane (DCYP), have been surveyed exercising the MEDT, reactivity indices, reactions, and activation energies, are computed. In an investigation of conceptual DFT indices, DCYP behaves as a nucleophile in this reaction, whereas ISALL acts as an electrophile. This cyclization is stereo-, chemo-, and regiospecific, as demonstrated by the activation and reaction energies, in clear agreement with the experiment’s results. The mechanism for this [3+2] cycloaddition is occurring in two steps, according to ELF analysis.
Methods
For the purposes of this investigation, all computations were performed using the Gaussian 09 program. The optimization was completed using Berny’s computational gradient optimization approach with the basis set 6-311G(d,p) and wB97XD functional. Frequency computations were utilized to characterize and locate stationary points where the transition phases have just one imaginary frequency and all frequencies for the reactants and products are positive. After evaluating the effect of dichloromethane (DCM) as a reaction solvent, the stationary point optimization was updated using the polarizable continuum model (PCM) developed by the Tomasi team. The electron localization function (ELF) has been examined within the context of topological investigations using Multiwfn software with a 0.05 grid step.
Graphical Abstract







Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
References
Holota S, Derkach H, Demchuk IL et al (2019) Synthesis and In vivo evaluation of pyrazoline-thiazolidin-4-one hybrid Les-5581 as a potential non-steroidal anti-inflammatory agent. Biopolym Cell 35(6). https://doi.org/10.7124/bc.000A17
Jasril THY, Aisyah N, Hendra R (2019) Microwave assisted synthesis and evaluation of toxicity and antioxidant activity of pyrazoline derivatives. Indones J Chem 19(3). https://doi.org/10.22146/ijc.34285
Ahmad A, Husain A, Khan SA, Mujeeb M, Bhandari A (2016) Synthesis, antimicrobial and antitubercular activities of some novel pyrazoline derivatives antimicrobial and antitubercular activities of novel pyrazoline derivatives. J Saudi Chem Soc 20(5). https://doi.org/10.1016/j.jscs.2014.12.004
Praceka MS, Megantara S, Maharani R, Muchtaridi M (2021) Comparison of various synthesis methods and synthesis parameters of pyrazoline derivates. J Adv Pharm Technol Res 12(4). https://doi.org/10.4103/japtr.JAPTR_252_21
Castro Sánchez ME, Noriega L, Perez-Aguilar JM, Caballero-Concha NA, Merino-Montiel P, López AR, Melendez Bustamante FJ (2022) 8 - Green chemistry approaches to the synthesis of pyrazoline steroid derivatives and their theoretical DFT characterization. Green Chemistry and Computational Chemistry 193–214. https://doi.org/10.1016/B978-0-12-819879-7.00008-8
Di M, Rein KS (2004) Aza analogs of kainoids by dipolar cycloaddition. Tetrahedron Lett 45(24). https://doi.org/10.1016/j.tetlet.2004.04.097
Tomilov YV, Revunov EV, Shulishov EV, Semenov VV (2012) 1,3-Dipolar addition of diazocyclopropane to eudesmane-type methylidene lactones and thermolysis of the resulting spiro-fused pyrazolines. Russ Chem Bull 61(2). https://doi.org/10.1007/s11172-012-0039-0
Domingo LR (2016) Molecular electron density theory: a modern view of reactivity in organic chemistry. Molecules 21(10). https://doi.org/10.3390/molecules21101319
Salah M, Belghiti ME, Aitouna AO, Zeroual A, Jorio S, El Alaoui Abdellaoui H, El Hadki H, Marakchi K, Komiha N (2021) MEDT study of the 1,3-DC reaction of diazomethane with Psilostachyin and investigation about the interactions of some pyrazoline derivatives with protease (Mpro) of nCoV-2. J Mol Graph Model 102:107763. https://doi.org/10.1016/j.jmgm.2020.107763
Fukui K (1997) A simple quantum-theoretical interpretation of the chemical reactivity of organic compounds. Frontier Orbitals And Reaction Paths: Selected Papers of Kenichi Fukui. https://doi.org/10.1142/9789812795847_0014
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21(6). https://doi.org/10.3390/molecules21060748
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92(9). https://doi.org/10.1063/1.458517
Thakkar AJ (2003) The momentum density perspective of the electronic structure of atoms and molecules. In: Rice SA (ed) Advances in chemical physics. https://doi.org/10.1002/0471484237.ch5
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132(18). https://doi.org/10.1021/ja100936w
Domingo LR, Ríos-Gutiérrez M, Emamian S (2017) Understanding the domino reaction between 1-diazopropan-2-one and 1,1-dinitroethylene. A molecular electron density theory study of the [3 + 2] cycloaddition reactions of diazoalkanes with electron-deficient ethylenes. RSC Adv 7(25). https://doi.org/10.1039/C7RA00544J
El Idrissi M, El Ghozlani M, Eşme A, Ríos-Gutiérrez M, Ouled Aitouna A, Salah M, El Abdallaoui HE, Zeroual A, Mazoir N, Domingo LR (2021) Mpro-SARS-CoV-2 inhibitors and various chemical reactivity of 1-bromo- and 1-chloro-4-vinylbenzene in [3+2] cycloaddition reactions. Organics 2(1):1–16. https://doi.org/10.3390/org2010001
Zeroual A, Ríos-Gutiérrez M, El Ghozlani M, El Idrissi M, Ouled Aitouna A, Salah M, El Abdallaoui HE (2020) Domingo LR (2020) A molecular electron density theory investigation of the molecular mechanism, regioselectivity, stereoselectivity and chemoselectivity of cycloaddition reaction between acetonitrile N-oxide and 2,5-dimethyl-2H-[1,2,3] diazarsole. Theor Chem Acc 139:37. https://doi.org/10.1007/s00214-020-2547-6
Zeroual A, Ríos-Gutiérrez M, Salah M, El Abdallaoui HE, Domingo LR (2019) An investigation of the molecular mechanism, chemoselectivity and regioselectivity of cycloaddition reaction between acetonitrile N-Oxide and 2,5-dimethyl-2H-[1,2,3]diazaphosphole: a MEDT study. J Chem Sci 131:75. https://doi.org/10.1007/s12039-019-1656-z
El Idrissi M, Eşme A, Hakmaoui Y, Ríos-Gutiérrez M, Ouled Aitouna A, Salah M, Zeroual A (2021) Domingo LR
(2021)Divulging the various chemical reactivity of trifluoromethyl-4-vinyl-benzene as well as methyl-4-vinyl-benzene in [3+2] cycloaddition reactions. J Mol Graph Model 102:107760. https://doi.org/10.1016/j.jmgm.2020.107760Mohammad-Salim HA, Basheer HA, Abdallah HH, Zeroual A (2021) Abdi Jamila L
(2021)A molecular electron density theory study for [3+2] cycloaddition reactions of N-benzylcyclohexylnitrone with methyl-3-butenoate. New J Chem 45:262–267. https://doi.org/10.1039/D0NJ04049ESalah M, Zeroual A, Jorio S, El Hadki H, Kabbaj O, Marakchi K, Komiha N (2020) Theoretical study of the 1,3-DC reaction between fluorinated alkynes and azides: reactivity indices, Transition structures, IGM and ELF analysis. J Mol Graph Model 94:107458. https://doi.org/10.1016/j.jmgm.2019.107458
Zeroual A, Benharref A, El Hajbi A (2015) Theoretical study of stereoselectivity of the [1+2] cycloaddition reaction between (1S,3R,8S)-2,2-dichloro-3,7,7,10-tetramethyltricyclo[6,4,0,01.3]dodec-9-ene and dibromocarbene using density functional theory (DFT) B3LYP/6-31G*(d). J Mol Model 21:44. https://doi.org/10.1007/s00894-015-2594-4
Barhoumi A, El Idrissi M, Zeroual A et al (2021) Theoretical study of the chemical reactivity of a class of trivalent phosphorus derivatives towards polyhaloalkanes: DFT study. J Mol Model 27:197. https://doi.org/10.1007/s00894-021-04814-0
El Ghozlani M, Barhoumi A, Elkacmi R, Ouled Aitouna A, Zeroual A, El Idrissi M (2020) Mechanistic study of hetero-Diels–Alder [4 + 2] cycloaddition reactions between 2-nitro-1H-pyrrole and isoprene. Chem Afr 3:901–909. https://doi.org/10.1007/s42250-020-00187-8
Barhoumi A, Ourhriss N, Elalaoui Belghiti M, Chafi M, Syed A, Eswaramoorthy R-M, Verma M, Zeroual A, Zawadzińska K, Jasiński R (2023) 3-Difluormethyl-5-carbomethoxy-2,4-pyrazole: Molecular mechanism of the formation and molecular docking study. Current Chemistry Letters 12:477–488. https://doi.org/10.5267/j.ccl.2023.3.008
Abdoul-Hakim M, El Idrissi K, Zeroual A, Garmes H (2023) Investigation of the solvent effect, regioselectivity, and the mechanism of the cycloaddition reaction between 2-chlorobenzimidazole and benzonitrile oxide. Chem Heterocycl Compd 59(3):155–164
Raji H, Ouled Aitouna A, Barhoumi A, Hammal R, Chekroun A, Zeroual A, Benharref A, Mazoir N (2023) [2+1] Cycloaddition reaction of α-atlantone with m-CPBA in the light of experimental and MEDT quantum-chemical study. Chem Heterocycl Compd 59(3):112–117
Ameur S, Barhoumi A, Ríos-Gutiérrez M, Ouled Aitouna A (2023) El Alaoui El Abdallaoui H, Mazoir N, Elalaoui Belghiti M, Syed A, Zeroual A, Domingo L R, A MEDT study of the mechanism and selectivity of the hetero-Diels–Alder reaction between 3-benzoylpyrrolo[1,2-c][1,4]-benzoxazine-1,2,4-trione and vinyl acetate. Chem Heterocycl Compd 59(3):165–170
Zeroual A, Ríos-Gutiérrez M, Amiri O, El Idrissi M, Domingo LR (2019) An MEDT study of the mechanism, chemo- and stereoselectivity of the epoxidation reaction of R-carvone with peracetic acid. RSC Adv-R Soc Chem 9:28500–28509
Zeroual A, Ríos-Gutiérrez M, El Idrissi M, Abdallaoui El Alaoui El, H, Domingo LR, (2019) An MEDT study of the mechanism and selectivities of the [3+2] cycloaddition reaction of tomentosin with benzonitrile oxide. Int J Quantum Chem 119:e25980. https://doi.org/10.1002/qua.25980
Zeroual A, Zoubir M, Hammal R, Benharref A, El Hajbi A (2015) Understanding the regioselectivity and reactivity of FriedeleCraftsbenzoylation using Parr function. Mor J Chem 3:356–360. https://doi.org/10.48317/IMIST.PRSM/morjchem-v3i4.3050
Ouahdi Z, Ourhriss N, Aitouna AO et al (2022) Exploration of the mechanism, chemospecificity, regiospecificity and stereoselectivity of the cycloaddition reaction between 9α-hydroxyparthenolide and nitrilimine: MEDT study. Theor Chem Acc 141:50. https://doi.org/10.1007/s00214-022-02913-6
Frisch MJ, Trucks GW, Schlegel HB et al (2016) Gaussian 09, Rev. D.01. Gaussian Inc, Wallingford, CT
Da CJ, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10(44). https://doi.org/10.1039/b810189b
Pople JA, Schleyer P V., Hehre WJ, Radom L. AB INITIO molecular orbital theory.; 1986. 10.1016/s0022-328x(00)99651-7
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3(2). https://doi.org/10.1002/jcc.540030212
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94(14). https://doi.org/10.1021/j100377a021
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94(7). https://doi.org/10.1021/cr00031a013
Domingo LR (2014) A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4(61). https://doi.org/10.1039/c4ra04280h
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3(5). https://doi.org/10.1039/c2ra22886f
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7). https://doi.org/10.1063/1.464913
Lu T, Chen F (2012) Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem 33(5). https://doi.org/10.1002/jcc.22885
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:9. https://doi.org/10.1021/ja983494x
Jaramillo P, Domingo LR, Chamorro E, Pérez P (2008) A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J Mol Struct Theochem:865(1-3). https://doi.org/10.1016/j.theochem.2008.06.022
Adjieufack AI, Ndassa IM, Ketcha Mbadcam J, Ríos-Gutiérrez M, Domingo LR (2017) Steric interactions controlling the syn diastereofacial selectivity in the [3 + 2] cycloaddition reaction between acetonitrile oxide and 7-oxanorborn-5-en-2-ones: a molecular electron density theory study. J Phys Org Chem 30(12). https://doi.org/10.1002/poc.3710
Raji H, Ouled Aitouna A, Barhoumi A, Chekroun A, Zeroual A, Syed A, Elgorban AM, Verma M, Benharref A, Varma RS (2023) Antiviral docking analysis, semisynthesis and mechanistic studies on the origin of stereo- and chemoselectivity in epoxidation reaction of α′-trans-Himachalene. J Mol Liq 385:122204. https://doi.org/10.1016/j.molliq.2023.122204
Asserne F, Ouahdi Z, Hakmaoui Y et al (2023) Molecular docking, regio, chemo and stereoselectivity study of the [3 + 2] cycloaddition reaction between pyridazi-3-one and nitrilimine. Chem Afr. https://doi.org/10.1007/s42250-023-00735-y
Ouahdi Z, Oueld Aitouna A, Barhoumi A, Belghiti ME, El Idrissi M, El Alaoui Abdellaoui H, Syed A, Zeroual A, Benharref A (2023) Elucidating the selectivities and the mechanism of [3+2] cycloloaddition reaction between 9α-hydroxyparthenolide and 4-methylbenzene-nitrile-oxide. Comput Theor Chem 1226:114212. https://doi.org/10.1016/j.comptc.2023.114212
Aitouna AO, Barhoumi A, Zeroual A (2023) A mechanism study and an investigation of the reason for the stereoselectivity in the [4+2] cycloaddition reaction between cyclopentadiene and gem-substituted ethylene electrophiles. Sci Rad 2(3):217–228. https://doi.org/10.58332/scirad2023v2i3a01
Funding
The authors extend their appreciation to the Researchers Supporting Project number (RSP2023R15), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Anas Ouled Aitouna, Ali Barhoumi, and Mohammed Elalaoui Belghiti: article writing. Abdellah Zeroual, Habib El Alaoui El Abdallaoui, and Noureddine Mazoir: numerical calculations and acquisition of data. Ali H. Bahkali and Meenakshi Verma: revising and editing language. Mohammed Elalaoui Belghiti Abdellah Zeroual and Asad Syed: final review and editing. All authors: analysis and interpretation of data and drafting the article.
Corresponding author
Ethics declarations
Ethics approval
The manuscript is prepared in compliance with the Ethics in Publishing Policy as described in the Guide for Authors.
Consent to participate
The manuscript is approved by all authors for publication.
Consent for publication
The consent for publication was obtained from all participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 1847 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ouled Aitouna, A., Barhoumi, A., El Alaoui El Abdallaoui, H. et al. Explaining the selectivities and the mechanism of [3+2] cycloloaddition reaction between isoalantolactone and diazocyclopropane. J Mol Model 29, 280 (2023). https://doi.org/10.1007/s00894-023-05688-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05688-0