Abstract
Context
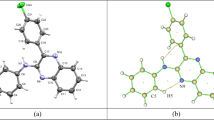
Theoretical investigation of indole (IND) and its binary combination with dichloromethane (DC) in various solvents were computed to track the impact of molecular interactions on spectral characteristics. When transitioning from plain drug to complexes, different modes of IND display a substantial shift in peak location. The 3561.26 cm−1 band shows (~15.58 cm−1) red shift upon dilution. The geometry in various solvents was calculated using quantum chemical calculation utilizing density functional theory (DFT). The highest ALIE values are located at the indole skeleton and on complexation with DC, and the ring atoms become more electron rich. The atom-centered density matrix propagation (ADMP) molecular dynamic (MD) calculation shows that the geometries optimized through the DFT calculation match the global minima effectively. MD simulations indicate that indole is more stable in water and methanol.
Methods
DFT studies have been employed to study the interaction between indole and dichloromethane. CAM-B3LYP/6-311++G(d)(6D,7F) level of theory was employed using Gaussian 16 W suite. Quantum topological descriptors were discussed using quantum theory of atoms in molecules (QTAIM) with the help of Multiwfn software. Reduced density gradient (RDG) plot describes the nature of the interaction, while average local ionization energy (ALIE) explained the variation in local ionization energy of the molecular surface before and after complexation.










Similar content being viewed by others
Data availability
The data used to support the findings of this study are included within the article.
Change history
27 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00894-023-05676-4
References
Wilson KR, Tobin JG, Ankudinov AL, Rehr JJ, Saykally RJ (2000) Extended X-ray absorption fine structure from hydrogen atoms in water. Phys Rev Lett 85:4289–4292. https://doi.org/10.1103/PhysRevLett.85.4289
Morgenstern K, Nieminen J (2002) Intermolecular bond length of ice on Ag(111). Phys Rev Lett 88:066102–066105. https://doi.org/10.1103/PhysRevLett.88.066102
Finney JL, Hallbrucker A, Kohl I, Soper AK, Bowron DT (2002) Structures of high and low density amorphous ice by neutron diffraction. Phys Rev Lett 88:225503–225506. https://doi.org/10.1103/PhysRevLett.8.225503
Hubbard RE, Haider MK, Muhammad Hydrogen bonds in proteins: role and strength, In: Encyclopedia of life sciences (ELS). John Wiley and Sons, Ltd: Chichester. https://doi.org/10.1002/97800470015902.a0003011.pub2
Scuderi D, Barbu-Debus KL, Zehnacker A (2011) The role of weak hydrogen bonds in chiral recognition. Phys Chem Chem Phys 13:17916–17929. https://doi.org/10.1039/C1CP20987F
Singh DK, Asthana BP, Srivastava SK (2012) Modeling the weak hydrogen bonding of pyrrole and dichloromethane through Raman and DFT study. J Mol Model 18:3541–3552. https://doi.org/10.1007/s00894-012-1355-x
Schlucker S, Singh RK, Asthana BP, Popp J, Kiefer W (2001) Hydrogen-bonded pyridine-water complexes studied by density functional theory and Raman spectroscopy. J Phys Chem A 105:9983–9989. https://doi.org/10.1021/jp0122272
Singh S, Srivastava SK, Singh DK (2013) Raman scattering and DFT calculation used for analyzing the structural features of DMSO in water and methanol. RSC Adv 3:4381–4390. https://doi.org/10.1039/C3RA22730H
Alves JEF, de Oliveira JF, deLima Souza TRC, de Moura RC, deCarvalho Junior LB, de Lima MCA, de Almeida SMV (2021) Novel indole-thiazole and indole-thiazolidinone derivatives as DNA groove binders. Int J Biol Macromol 170:622–635. https://doi.org/10.1016/j.ijbiomac.2020.12.153
Kochanoswka-Karamyan AJ, Hamann MT (2010) Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem Rev 110:4489–4497. https://doi.org/10.1021/cr900211p
Zhang MZ, Chen Q, Yang GF (2015) A review on recent developments of indole containing antiviral agents. Eur J Med Chem 89:421–441. https://doi.org/10.1016/j.ejmech.2014.10.065
Chen M, Gan L, Lin S, Wang X, Li L, Li Y, Zhu C, Wang Y, Jiang B, Jiang J, Yang Y, Shi J (2012) Alkaloids from the root of Isatis indigotica. J Nat Prod 75:1167–1176. https://doi.org/10.1021/np3002833
de Sa Alves FR, Barreiro EJ, Fraga CAM (2009) From nature to drug discovery: the indole scaffold as a privileged structure. Mini Rev Med Chem 9:782–793. https://doi.org/10.2174/138955709788452649
Almagro L, Fernandez-Perez F, Pedreno MA (2015) Indole alkaloids from catharanthus roseus: bioproduction and their effect on human health. Molecules 20:2973–3000. https://doi.org/10.3390/molecules20022973
Kaushik NK, Kaushik N, Attri P, Kumar N, Kim CH, Verma AK, Choi EH (2013) Biomedical importance of indoles. Molecules 18:6620–6662. https://doi.org/10.3390/molecules18066620
Welsch ME, Snyder SA, Stockwell BR (2010) Privileged scaffolds for library design and drug discovery. Curr Opin Chem Biol 14:347–361. https://doi.org/10.1016/j.cbpa.2010.02.018
Lal S, Snape TJ (2012) 2-Arylindoles: a privileged molecular scaffold with potent, broad ranging pharmacological activity. Curr Med Chem 19:4828–4837. https://doi.org/10.2174/092986712803341449
Patel DM, Sharma MG, Vala RM, Lagunes I, Puerta A, Padron JM, Rajani DP, Patel HM (2019) Hydroxyl alkyl ammonium ionic liquid assisted green and one port regioselective access to functionalized pyrazolodihydropyridine core and their pharmacological evaluation. Bioorg Chem 86:137–150. https://doi.org/10.1016/j.bioorg.2019.01.029
Shimazaki Y, Yajima T, Takani M, Yamauchi O (2009) Metal complexes involving indole rings: structures and effects of metal-indole interactions. Coord Chem Rev 253:479–492. https://doi.org/10.1016/j.ccr.2008.04.012
Srivastava N, Banki BK (2003) Bismuth nitrate-catalyzed versatile Michael reactions. J Org Chem 68:2109–2114. https://doi.org/10.1021/jo026550s
Geetha DV, Hezam AF, Hussein EMY, Sridhar MA, Shaukath AD, Loknath NK (2019) Synthesis, elucidation, Hirshfeld surface analysis and DFT calculations of 4-chloro-N-[2-(2-1H-indol-3-yl-acetylamino)-pheny]-benzamide. J Mol Struct 1178:384–393. https://doi.org/10.1016/j.molstruc.2018.10.016
Gupta PSS, Bhat HR, Biswal S, Rana MK (2020) Computer aided discovery of bis-indole derivatives as multitarget drugs against cancer and bacterial infections: DFT, docking, virtual screening and molecular dynamics studies. J Mol Liq 320:114375. https://doi.org/10.1016/j.molliq.2020.114375
Gaussian 16, Revision A.03, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian, Inc., Wallingford CT.
GaussView, Version 6.1, Dennington R, Keith TA, Millam JM (2016) Semichem Inc., Shawnee Mission, KS.
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Mennucci B, Tomasi J (1997) Continuum solvation models: a new approach to the problem of solute’s charge distribution and cavity boundaries. J Chem Phys 106:5151–5158. https://doi.org/10.1063/1.473558
Mohankumar T, Chandramohan V, Lalithamba HS, Jayaraj RL, Kumardhas P, Sivandam M, Hunday G, Vijayakumar R, Balakrishnan R, Manimaran D, Elangovan N (2020) Design and molecular dynamic investigations of 7,8-dihydroxyflavone derivatives as potential neuroprotective agents against alpha-synuclein. Sci Rep 10:599. https://doi.org/10.1038/s41598-020-57417-9
Kpemissi M, Potarniche AV, Lawson-Evi P, Metowogo K, Mella M, Dramane P, Taulescu M, Chandramohan V, Suhas DS, Puneeth TA, Kumar SV, Vlase L, Andrei S, Eklu-Gadgbeku K, Sevastre B, Veerapur VP (2020) Nephroprotective effect of combretum micranthum G.Don in nicotinamide-streptozotocin induced diabetic nephropathy in rats: in vivo and in silico experiments. J. Ethnopharmacol 261:113133. https://doi.org/10.1016/j.jep.2020.113133
Kpemissi M, Eklu-Gadegbeku K, Veerapur VP, Negru M, Taulescu M, Chandramohan V, Hiriyan J, Banakar SM, Nv T, Suhas DS, Puneeth TA, Vijayakumar S, Metowogo K, Akilkokou K (2019) Nephroprotective activity of combretum micranthum G. Don in cisplatin induced nephrotoxicity in rats: in vitro, in-vivo and in-silico experiments. Biomed Pharmacother 116:108961. https://doi.org/10.1106/j.biopha.2019.108961
Al-Otaibi JS, Mary YS, Mary YS, Panicker CY, Thomas R (2019) Cocrystals of pyrazinamide with p-toluenesulfonic and ferulic acids: DFT investigations and molecular docking studies. J Mol Struct 1175:916–926. https://doi.org/10.1016/j.molstruc.2018.08.055
Mary YS, Mary YS (2021) Utilization of doped/undoped graphene quantum dots for ultrasensitive detection of duphaston, a SERS platform. Spectrochim Acta A 244:118865. https://doi.org/10.1016/j.saa.2020.118865
Almuqrin AH, Al-Otaibi JS, Mary YS, Mary YS (2021) DFT computational study towards investigating psychotropic drugs, promazine and trifluoperazine adsorption on graphene, fullerene and carbon cyclic ring nano clusters. Spectrochim Acta A 246:119012. https://doi.org/10.1016/j.saa.2020.119012
Al-Otaibi JS, Mary YS, Mary YS, Thomas R (2019) Quantum mechanical and photovoltaic studies on the cocrystals of hydrochlorothiazide with isonazid and malonamide. J Mol Struct 1197:719–726. https://doi.org/10.1016/j.molstruc.2019.07.110
Al-Otaibi JS, Mary YS, Mary YS, Serdaroglu G (2021) Adsorption of adipic acid in Al/B-N/P nanocages: DFT investigations. J Mol Mod 27:113. https://doi.org/10.1007/s00894-021-04742-z
Smitha M, Mary YS, Mary YS, Serdaroglu G, Chowdhury P, Rana M, Umamahesvari H, Sarojini BK, Mohan BJ, Pavithran R (2021) Modeling the DFT structural and reactivity studies of a pyrimidine-6-carboxylate derivative with reference to its wavefunction dependent, MD simulations and evaluation for potential antimicrobial activity. J Mol Struct 1237:130397. https://doi.org/10.1016/j.molstruc.2021.130397
Al-Otaibi JS, Mary YS, Armakovic SJ, Thomas R (2020) Hybrid and bioactive cocrystals of pyrazinamide with hydroxybenzoic acids: detailed study of structure, spectroscopic characteristics, other potential applications and noncovalent interactions using SAPT. J Mol Struct 1202:127316. https://doi.org/10.1016/j.molstruc.2019.127316
Mary YS, Mary YS, Armakovic S, Armakovic SJ, Yadav R, Celik I, Mane P, Chakraborty B (2021) Stability and reactivity study of bio-molecules brucine and colchicines towards electrophile and nucleophile attacks: insight from DFT and MD simulations. J Mol Liq 335:116192. https://doi.org/10.1016/j.molliq.2021.116192
Mary YS, Mary YS, Bielenica A, Armakovic S, Armakovic SJ, Chandramohan V, Dammalli M (2021) Investigation of the reactivity properties of a thiourea derivative with anticancer activity by DFT and MD simulations. J Mol Model 27:217. https://doi.org/10.1007/s00894-021-04835-9
Mary YS, Mary YS, Ullah Z (2021) Computational study of sorbic acid drug adsorption onto coronene/fullerene/fullerene-like X12Y12 (X=Al, B and Y=N,P) nanocages: DFT and molecular docking investigations. J Cluster Sci. https://doi.org/10.1007/s10876-021-02106-4
Mary YS, Mary YS, Armakovic S, Armakovic SJ, Yadav R, Celik I, Razavi R (2021) Investigation of reactive properties, adsorption on fullerene, DFT, Molecular dynamics simulation of an anthracene derivative targeting dihydrofolate reductase and human dUTPase. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2021.1953602
Mary YS, Mary YS, Chandramohan V, Kumar N, Van Alsenoy C, Gamberini MC (2020) DFT and MD simulations and molecular docking of cocrystals of octafluoro-1,4-diiodobutane with phenazine and acridine. Struct Chem 31:2525–2531. https://doi.org/10.1007/211224-020-01616-7
Murray JS, Seminario JM, Politzer P, Sjoberg P (1990) Average local ionization energies computed on the surfaces of some strained molecules. Int J Quantum Chem 38:645–653. https://doi.org/10.1002/qua.5603
Alsalme A, Pooventhiran T, Al-Zaqri N, Rao DJ, Thomas R (2021) Structural, physic-chemical landscapes, ground state and excited state properties in different solvents atmosphere of avapritinib and its ultrasensitive detection using SERS/GERS on self assembly formation with graphene quantum dots. J Mol Liquid 322:114555. https://doi.org/10.1016/j.molliq.2020.114555
Sjoberg P, Murray JS, Brinck T, Politzer P (1990) Average local ionization energies on the molecular surfaces of aromatic systems as guides to chemical reactivity. Can J Chem 68:1440–1443. https://doi.org/10.1139/v90-220
Politzer P, Abu-Awwad F, Murray JS (1998) Comparison of density functional and Hartree-Fock average local ionization energies on molecular surfaces. Int J Quantum Chem 69:607–613. https://doi.org/10.1002/(SICI)1097-461X(1998)69:4<607::AID-QUA18>3.0.CO:2-W
Surendar P, Pooventhiran T, Rajam S, Bhattacharyya U, Bakht A, Thomas R (2021) Quasi-liquid Schiff bases from trans-2-hexenal and cytosine and l-leucine with potentialantieczematic and antiarthritic activities: synthesis, structure and quantum mechanical studies. J Mol Liq 334:116448. https://doi.org/10.1016/j.molliq.2021.116448
Alharthi FA, Al-Zaqri N, Alsalme A, Al-Taleb A, Pooventhiran T, Thomas R, Rao DJ (2021) Excited state electronic properties, structural studies, noncovalent interactions and inhibition of the novel severe acute respiratory syndrome corona virus 2 proteins in ripretinib by first principle simulations. J Mol Liq 324:115134. https://doi.org/10.1016/j.molliq.2021.115134
Politzer P, Murray JS, Bulat FA (2010) Average local ionization energy: a review. J Mol Model 16:1731–1742. https://doi.org/10.1007/s00894-010-0709-5
Funding
The authors express their gratitude to Princess Nourah Bint Abdulrahman University Researchers Supporting Project (number PNURSP2023R1), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Jamelah S. Al-Otaibi: software, supervision, manuscript preparation, and data analysis. Y. Sheena Mary: supervision, manuscript preparation, conceiving the problem, and data analysis. Y. Shyma Mary: manuscript preparation, conceiving the problem, and data analysis and correction. Renjith Thomas: manuscript preparation, conceiving the problem, and data analysis and correction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to the changes of year in Funding section.
Supplementary information
ESM 1
(DOCX 173 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Otaibi, J.S., Mary, Y.S., Mary, Y.S. et al. Evidences of noncovalent interactions between indole and dichloromethane under different solvent conditions. J Mol Model 29, 246 (2023). https://doi.org/10.1007/s00894-023-05623-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05623-3