Abstract
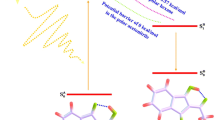
In this work, we were devoted to explore the effect of solvent polarities on the excited-state intramolecular proton transfer (ESIPT) process of 1-acetamido-4-hydroxyanthraquinone (AcHAQ) in three different polarity solvents (acetonitrile, chloroform, and cyclohexane) based on the density functional theory (DFT) and time-dependent DFT (TD-DFT) methods, and thereby regulating the distribution ratio between the dual excited-state isomers (enol and keto). The calculated geometrical parameters and infrared (IR) vibrational spectra have confirmed the excited-state intramolecular hydrogen bond (IHB) strengthening mechanism. Natural bond orbital (NBO) population analysis indicates that the intramolecular charge transfer (ICT) around IHBs has enhanced IHB, thereby triggering the ESIPT reaction. In addition, results obtained from the scanned potential energy curve (PEC) manifest that ESIPT process prefers to occur along the O3-H2…O1 IHB and energy barriers corresponding to the proton transfer in ACN are the lowest among all the studied solvents.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Tang KC, Chang MJ, Lin TY et al (2011) J Am Chem Soc 133:17738
Chen L, Ye JW, Wang HP et al (2017) Nat Commun 8:15985
Yang XF, Huang Q, Zhong YG et al (2014) Chem Sci 5:2177
Wu LL, Sedgwick AC, Sun XL, Bull SD, He XP, James TD (2019) Acc Chem Res 52:2582
Padalkar VS, Seki S (2016) Chem Soc Rev 45:169
Zhang W, Yan YL, Gu JM, Yao JN, Zhao YS (2015) Angew Chem Int Ed 54:7125
Kim D, Jeong K, Kwon JE et al (2019) Nat Commun 10:3089
Wei GQ, Yu Y, Zhuo MP, Wang XD, Liao LS (2020) J Mater Chem C 8:11916
Qian HL, Dai C, Yang CX, Yan XP (2017) ACS Appl Mater Interfaces 9:24999
Boonkitpatarakul K, Wang JF, Niamnont N et al (2016) ACS Sens 1:144
Azarias C, Budzak S, Laurent AD, Ulrich G, Jacquemin D (2016) Chem Sci 7:3763
Tian MG, Ma YY, Lin WY (2019) Acc Chem Res 52:2147
Shang CJ, Cao YJ, Sun CF, Zhao HF (2022) Phys Chem Chem Phys 24:8453
Dutta S, Mandal D (2022) J Mol Liq 361:119651
Han JH, Cao BF, Li Y et al (2020) Spectrochim Acta, Part A 231:118086
Cao YJ, Yu XR, Sun CF, Cui JA (2022) Int J Mol Sci 23:2132
Niu YH, Wang R, Shao PL, Wang YX, Zhang YR (2018) Chem - Eur J 24:16670
Yang YF, Luo X, Ma FC, Li YQ (2021) Spectrochim Acta, Part A 250:119375
Sun CF, Li Y, Li B et al (2020) J Mol Liq 297:111937
Sun CF, Zhao HF, Liu XC, Yin H, Shi Y (2018) Org Chem Front 5:3435
Yang WY, Lai RC, Wu JJ et al (2022) Adv Funct Mater 32:2204129
Zhang YJ, Shang CJ, Cao YJ, Ma M, Sun CF (2022) Spectrochim Acta, Part A 280:121559
Li Q, Wan Y, Zhou Q et al (2022) Spectrochim Acta, Part A 272:120953
Yu XR, Cao YN, Li YZ, Cui JA, Sun CF (2022) J Mol Struct 1250:131923
Tirado-Rives J, Jorgensen WL (2008) J Chem Theory Comput 4:297
Cossi M, Barone V (2001) J Chem Phys 115:4708
Adamo C, Jacquemin D (2013) Chem Soc Rev 42:845
Zhang J, Lalevee J, Hill NS et al (2018) Macromolecules 51:8165
Durant JL (1996) Chem Phys Lett 256:595
Jacquemin D, Wathelet V, Perpete EA, Adamo C (2009) J Chem Theory Comput 5:2420
Jacquemin D, Perpete EA, Scuseria GE, Ciofini I, Adamo C (2008) J Chem Theory Comput 4:123
Valdes H, Pluhackova K, Pitonak M, Rezac J, Hobza P (2008) Phys Chem Chem Phys 10:2747
Zhao JF, Chen JS, Liu JY, Hoffmann MR (2015) Phys Chem Chem Phys 17:11990
Zhou PW, Han K (2018) Acc Chem Res 51:1681
Yin H, Li H, Xia GM et al (2016) Sci Rep 6:19774
Li CZ, Ma C, Li DL, Liu YF (2016) J Lumin 172:29
Li YQ, Ma YZ, Yang YF, Shi W, Lan RF, Guo Q (2018) Phys Chem Chem Phys 20:4208
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams DF, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16. Gaussian Inc, Wallingford, CT, USA
Liang NQ, Kuwata S, Ishige R, Ando S (2021) Mater Chem Front 6:24
Li Y, Sun CF, Han JH et al (2020) J Lumin 221:117110
Inamdar SR, Mannekutla JR, Sannaikar MS, Wari MN, Mulimani BG, Savadatti MI (2018) J Mol Liq 268:66
Funding
This work was financially supported by the Innovation Training Project Program of Heilongjiang Province (No. S202210225053) and the Fundamental Research Funds for the Central Universities (No. 2572020BC03).
Author information
Authors and Affiliations
Contributions
Xin Xu: conceptualization, data curation, writing—original draft. Zeran Zhang: investigation, writing—review and editing. Yajie Zhang, Linyue Jin, and Qian Cheng: writing—review and editing. Fang Liu: conceptualization, methodology, writing—review and editing, resources. Chaofan Sun: conceptualization, methodology, investigation, software, writing—review and editing, resources.
Corresponding authors
Ethics declarations
Consent for publication
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rights holder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, X., Zhang, Z., Zhang, Y. et al. Theoretically unveiling the effect of solvent polarities on ESDPT mechanisms and photophysical properties of hydroxyanthraquinones. J Mol Model 28, 389 (2022). https://doi.org/10.1007/s00894-022-05383-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05383-6