Abstract
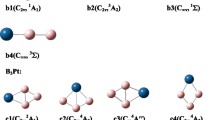
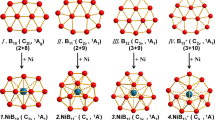
In this study, we provide a theoretical evaluation of relative stabilities and electronic structure for [BnXn]2− clusters (n = 10, 12, 13, 14, 15, 16). Structural and electronic characteristics of [BnXn]2− clusters are examined by comparison with the [B12X12]2− counterparts with a focus on the substituent effects (X = H, F, Cl, Br, CN, BO, OH, NH2) on the electronic structure, electron detachment energies, formation enthalpies, and charge distributions. For the electronic structure and electron detachment energies, substituent effects on boron clusters are shown to follow a very similar trend to the mesomeric and inductive effects (± M and ± I) of π-conjugated systems, and the most stable derivatives in terms of HOMO/LUMO and electron detachment energies are calculated for CN and BO substituents due to strong -M effects. In the case of formation enthalpies for larger boron clusters (n ≥ 13), the icosahedral barrier is shown to increase with the halogen and CN substitution, whereas it is possible to reduce the icosahedral barrier for the cases of X = OH and NH2. It is shown that this reduction results from destabilizing the [B12X12]2− cluster with electronic (+ M) and symmetry effects induced by OH and NH2 ligands.







Similar content being viewed by others
Availability of data and material
All relevant data are available in the electronic supplementary information.
Code availability
Gaussian 09 Revision A.02 is used for all the calculations in this work. Gaussview 5.0 is used for the visualization and generation of figures in the article.
References
Gonzalez Szwacki N, Sadrzadeh A, Yakobson BI (2007) B80 fullerene: an ab initio prediction of geometry, stability, and electronic structure. Phys Rev Lett 98(16):166804
Cheng L (2012) B14: an all-boron fullerene. J Chem Phys 136(10):104301
Shen YF, Xu C, Cheng LJ (2017) Deciphering chemical bonding in BnHn2- (n = 2–17): flexible multicenter bonding. RSC Adv 7:36755–36764
Lipscomb WN, Epstein IR (1971) Boron hydride valence structures. Topological approach. Inorg Chem 10(9):1921–1928
Bicerano J, Marynick DS, Lipscomb WN (1978) Molecular orbital studies on large closo boron hydrides. Inorg Chem 17(12):3443–3453
Williams RE (1992) The polyborane, carborane, carbocation continuum: architectural patterns. Chem Rev 92(2):177–207
Schleyer PR, Najafian K, Mebel AM (1998) The large closo-borane dianions, B(n)H(n)(2-) (n = 13–17) are aromatic, why are they unknown? Inorg Chem 37(26):6765–6772
Brown LD, Lipscomb WN (1977) Closo boron hydrides with 13 to 24 boron atoms. Inorg Chem 16(12):2989–2996
Grimes RN (2004) Boron Clusters Come of Age. J Chem Educ 81(5)
Byun D et al (1994) Photoemission from gaseous and condensed molecular carborane cluster molecules. J Electron Spectrosc Relat Phenom 69(2):111–116
Fang H, Jena P (2019) Stable tetra- and penta-anions in the gas phase. Angew Chem Int Ed Engl 58(33):11248–11252
Joshi M, Ghanty TK (2019) Lanthanide and actinide doped B12H12(2-) and Al12H12(2-) clusters: new magnetic superatoms with f-block elements. Phys Chem Chem Phys 21(42):23720–23732
Lee S et al (1992) Structures of selected boranes and carboranes. J Vac Sci Technol A 10(4):881–885
Pathak B et al (2011) Borane derivatives: a new class of super- and hyperhalogens. ChemPhysChem 12(13):2423–2428
Zahradnik R, Balaji V, Michl J (1991) An SCF study of 10-vertex and 12-vertex boranes and heteroboranes. J Comput Chem 12(9):1147–1156
Giri S, Behera S, Jena P (2014) Superhalogens as building blocks of halogen-free electrolytes in lithium-ion batteries. Angew Chem Int Ed 53(50):13916–13919
Tutusaus O et al (2015) An efficient halogen-free electrolyte for use in rechargeable magnesium batteries. Angew Chem Int Ed Engl 54(27):7900–7904
Zhong M et al (2017) Role of ligands in the stability of BnXn and CBn-1Xn (n = 5–10; X = H, F, CN) and their potential as building blocks of electrolytes in lithium ion batteries. Phys Chem Chem Phys 19(27):17937–17943
Maderna A, Knobler CB, Hawthorne MF (2001) Twelvefold functionalization of an icosahedral surface by total esterification of [B12(OH)12]2-: 12(12)-closomers. Angew Chem Int Ed Engl 40(16):2947
Apra E et al (2019) A benchmark photoelectron spectroscopic and theoretical study of the electronic stability of [B12H12](2). J Chem Phys 150(16):164306
Lipscomb WN, Pitochelli AR, Hawthorne FM (1959) PROBABLE STRUCTURE OF THE B10H10-2 ION. J Am Chem Soc 81(21):5833–5834
Kaczmarczyk A, Dobrott RD, Lipscomb WN (1962) REACTIONS OF B10H10-2 ION. Proc Natl Acad Sci U S A 48(5):729–733
Klanberg F, Muetterties EL (1966) Chemistry of Boranes. XXVII. New polyhedral borane anions, B9H92- and B11H112-. Inorg Chem 5(11):1955–1060
Klanberg F et al (1967) Chemistry of boranes. XXVIII. New polyhedral borane anions, B8H82-, B8H8.-, and B7H72-. Inorg Chem 6(7):1271–1281
Wade K (1971) The structural significance of the number of skeletal bonding electron-pairs in Carboranes, the higher boranes and borane anions, and various transition-metal carbonyl cluster compounds. J Chem Soc D 15:792–793
Wade K (1976) Structural and bonding patterns in cluster chemistry. Adv Inorganic Chem Radiochem 18:1–66
Mingos DMP (1984) Polyhedral Skeletal Electron Pair Approach. Acc Chem Res 17(9):311–319
Chen G et al (2019) Rational design of stable dianions and the concept of super-chalcogens. J Phys Chem A 123(27):5753–5761
Burke A et al (2003) Beyond the icosahedron: the first 13-vertex carborane. Angew Chem Int Ed Engl 42(2):225–228
Zhang J, Xie Z (2014) Synthesis, structure, and reactivity of 13- and 14-vertex carboranes. Acc Chem Res 47(5):1623–1633
Deng L, Chan HS, Xie Z (2006) Synthesis, structure, and reactivity of 13-vertex carboranes and 14-vertex metallacarboranes. J Am Chem Soc 128(15):5219–5230
Zheng F et al (2020) Synthesis and X-ray characterization of 15- and 16-vertex closo-carboranes. Nat Commun 11(1):5943
Deng L et al (2006) Synthesis and structure of 14- and 15-vertex ruthenacarboranes. Angew Chem Int Ed Engl 45(26):4309–4313
Deng L, Xie Z (2007) A Journey from 12-vertex to 14-vertex carboranes and to 15-vertex metallacarboranes. Organometallics 26:1832–1845
Dreuw A, Zint N, Cederbaum LS (2002) Dianionic tetraborates do exist as stable entities. J Am Chem Soc 124(36):10903–10910
Zint N, Dreuw A, Cederbaum LS (2002) Gas-phase stability of derivatives of the closo-hexaborate dianion B6H62-. J Am Chem Soc 124(17):4910–4917
Boyd LA et al (2004) Exo-pi-bonding to an ortho-carborane hypercarbon atom: systematic icosahedral cage distortions reflected in the structures of the fluoro-, hydroxy- and amino-carboranes, 1-X-2-Ph-1,2–C2B10H10 (X=F, OH or NH2) and related anions. Dalton Trans 17:2786–2799
Warneke J et al (2017) Electronic structure and stability of [B12X12](2-) (X = F-At): a combined photoelectron spectroscopic and theoretical study. J Am Chem Soc 139(41):14749–14756
Zhao HM, Zhou J, Jena P (2016) Stability of B-12(CN)(12)(2-): Implications for lithium and magnesium ion batteries. Angew Chem Int Ed 55(11):3704–3708
Moon J, Baek H, Kim J (2018) Unusually high stability of B12(BO)122− achieved by boronyl ligand manipulation: theoretical investigation. Chem Phys Lett 698:72–76
Boere RT et al (2014) On the oxidation of the three-dimensional aromatics [B(12)X(12)](2-) (X=F, Cl, Br, I). Chemistry 20(15):4447–4459
Lee TB, McKee ML (2012) Redox energetics of hypercloso boron hydrides B(n)H(n) (n = 6–13) and B12X12 (X = F, Cl, OH, and CH3). Inorg Chem 51(7):4205–4214
Teixidor F, Vinas C (2018) Halogenated icosahedral carboranes: a platform for remarkable applications, in Handbook of Boron Science: With Applications in Organometallics, Catalysis, Materials and Medicine, N.S. Hosmane and R. Eagling, Editors. World Scientific
Axtell JC et al (2018) Synthesis and applications of perfunctionalized boron clusters. Inorg Chem 57(5):2333–2350
Grimes RN (2016) Carboranes. Academic Press, p 1058
Nunez R et al (2016) Electrochemistry and photoluminescence of icosahedral carboranes, boranes, metallacarboranes, and their derivatives. Chem Rev 116(23):14307–14378
Avelar A, Tham FS, Reed CA (2009) Superacidity of boron acids H2(B12X12) (X = Cl, Br). Angew Chem Int Ed Engl 48(19):3491–3493
Frisch MJ et al (2009) Gaussian 09. Gaussian Inc, Wallingford CT
Dennington RD, Keith TA, Milliam JM (2009) GaussView 5.0. Semichem Inc., Wallingford CT
Perdew JP, Ernzerhof M, Burke K (1996) Rationale for mixing exact exchange with density functional approximations. J Chem Phys 105(22):9982–9985
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: The PBE0 model. J Chem Phys 110(13):6158–6170
Perdew JP (1986) Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B Condens Matter 33(12):8822–8824
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A Gen Phys 38(6):3098–3100
Becke AD (1993) Density-functional thermochemistry. 3. The role of exact exchange. J Chem Phys 98(7):5648–5652
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theoret Chem Acc 120(1–3):215–241
Breneman CM, Wiberg KB (1990) Determining atom-centered monopoles from molecular electrostatic potentials - the need for high sampling density in formamide conformational-analysis. J Comput Chem 11(3):361–373
Foster JP, Weinhold F (1980) Natural Hybrid Orbitals. J Am Chem Soc 102(24):7211–7218
Reed AE, Weinhold F (1983) Natural Bond Orbital Analysis of near-Hartree-Fock Water Dimer. J Chem Phys 78(6):4066–4073
Reed AE, Weinhold F (1985) Natural Localized Molecular-Orbitals. J Chem Phys 83(4):1736–1740
Reed AE, Weinstock RB, Weinhold F (1985) Natural-Population Analysis. J Chem Phys 83(2):735–746
Zhang JJ, Lin ZY, Xie ZW (2015) DFT Studies on structures, stabilities, and electron affinities of closo-supercarboranes C2Bn-2Hn (n=13-20). Organometallics 34(23):5576–5588
Liao RB et al (2015) A topological pattern for understanding the structures of boranes and borane analog compounds. Struct Chem 26(1):353–364
Krygowski TM, Stepien BT (2005) Sigma- and pi-electron delocalization: focus on substituent effects. Chem Rev 105(10):3482–3512
Krygowski TM et al (2014) Aromaticity from the viewpoint of molecular geometry: application to planar systems. Chem Rev 114(12):6383–6422
Milian Medina B et al (2007) Effect of fluorination on the electronic structure and optical excitations of pi-conjugated molecules. J Chem Phys 126(11):111101
Fanfrlik J et al (2006) Interaction of carboranes with biomolecules: formation of dihydrogen bonds. ChemPhysChem 7(5):1100–1105
Klooster WT et al (1999) Study of the N−H···H−B dihydrogen bond including the crystal structure of BH3NH3 by neutron diffraction. J Am Chem Soc 121(27):6337–6343
Li J, Zhao F, Jing F (2002) B-Hδ−σ bond as dihydrogen bond acceptor: some theoretical observations and predictions. J Chem Phys 116(1):25–32
Lyssebko KA, Antipin MY (2004) Nature of weak inter- and intramolecular interactions in crystals. 1. The F...O and F...H contacts in the crystal of 2-trifluoroacetyl-5-trifluoromethylpyrrole. Russian Chem Bull 53:10–17
Planas JG et al (2005) Self-assembly of mercaptane-metallacarborane complexes by an unconventional cooperative effect: a C-H…S-H…H-B hydrogen/dihydrogen bond interaction. J Am Chem Soc 127(45):15976–82
Shubina ES et al (1998) Problems of unusual hydrogen bonds between proton donors and transition metal hydrides and borohydrides. Russ Chem Bull 47:817–822
Acknowledgements
We would like to thank Prof. Hakan Usta for carefully reading the manuscript and their valuable recommendations. We are indebted to Abdullah Gül University for making the facilities of the High-Performance Computing (HPC) center available for our computational work.
Funding
This work was supported by Research Fund of Abdullah Gül University (Project Number: FDK-2018–122).
Author information
Authors and Affiliations
Contributions
DT, FA, and MD conceived the idea and planned the computations in this study. DT ran the most of the computations and wrote the first draft for this manuscript. FA supervised the project. MD edited and reviewed the manuscript prior to submission. All authors approved the contents of this manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tahaoğlu, D., Alkan, F. & Durandurdu, M. Theoretical investigation of substituent effects on the relative stabilities and electronic structure of [BnXn]2− clusters. J Mol Model 27, 365 (2021). https://doi.org/10.1007/s00894-021-04980-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04980-1