Abstract
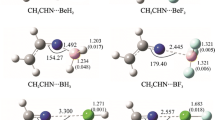
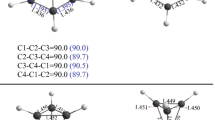
The properties of some types of noncovalent interactions formed by triplet diphenylcarbene (DPC3) have been investigated by means of density functional theory (DFT) calculations and quantum theory of atoms in molecule (QTAIM) studies. The DPC3···LA (LA = AlF3, SiF4, PF5, SF2, ClF) complexes have been analyzed from their equilibrium geometries, binding energies, and properties of electron density. The triel bond in the DPC3···AlF3 complex exhibits a partially covalent nature, with the binding energy − 65.7 kJ/mol. The tetrel bond, pnicogen bond, chalcogen bond, and halogen bond in the DPC3···LA (LA = SiF4, PF5, SF2, ClF) complexes show the character of a weak closed-shell noncovalent interaction. Polarization plays an important role in the formation of the studied complexes. The strength of intermolecular interaction decreases in the order LA = AlF3 > ClF > SF2 > SiF4 > PF5. The electron spin density transfers from the radical DPC3 to ClF and SF2 in the formation of halogen bond and chalcogen bond, but for the DPC3···AlF3/SiF4/PF5 complexes, the transfer of electron spin density is minimal.







Similar content being viewed by others
Data availability
The manuscript has full control of all primary data, and the authors agree to allow the journal to review their data if requested.
References
Müller-Dethlefs K, Hobza P (2000) Noncovalent interactions: a challenge for experiment and theory. Chem Rev 100:143–168
Gilli G, Gilli P (2009) The nature of the hydrogen bond: outline of a comprehensive hydrogen bond theory. Oxford University Press
Scheiner S (2019) Forty years of progress in the study of the hydrogen bond. Struct Chem 30:1119–1128
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Halogen bonding based recognition processes: a world parallel to hydrogen bonding. Acc Chem Res 38:386–395
Wang H, Wang W, Jin WJ (2016) σ-Hole bond vs π-hole bond: a comparison based on halogen bond. Chem Rev 116:5072–5104
Clark T, Hennemann M, Murray JS, Politzer P (2007) Halogen bonding: the σ-hole. J Mol Model 13:291–296
Murray JS, Lane P, Clark T, Riley KE, Politzer P (2012) σ-Holes, π-holes and electrostatically-driven interactions. J Mol Model 18:541–548
Politzer P, Murray JS (2013) Halogen bonding: an interim discussion. ChemPhysChem 14:278–294
Politzer P, Murray JS, Clark T (2013) Halogen bonding and other σ-hole interactions: a perspective. Phys Chem Chem Phys 15:11178–11189
Politzer P, Murray JS, Clark T, Resnati G (2017) The σ-hole revisited. Phys Chem Chem Phys 19:32166–32178
Politzer P, Murray JS (2019) An overview of strengths and directionalities of noncovalent interactions: σ-holes and π-holes. Crystals 9:165
Politzer P, Murray JS (2020) Electrostatics and polarization in σ- and π-hole noncovalent interactions: an overview. ChemPhysChem 21:579–588
Yáñez M, Sanz P, Mó O, Alkorta I, Elguero J (2009) Beryllium bonds, do they exist? J Chem Theor Comput 5:2763–2771
Grabowski SJ (2014) Boron and other triel Lewis acid centers: from hypovalency to hypervalency. ChemPhysChem 15(14):2985–2993
Gao L, Zeng Y, Zhang X, Meng L (2016) Comparative studies on group III σ-hole and π-hole interactions. J Comput Chem 37(14):1321–1327
Bauzá A, Mooibroek TJ, Frontera A (2013) Tetrel-bonding interaction: rediscovered supramolecular force? Angew Chem Int Ed 52(47):12317–12321
Zahn S, Frank R, Hey-Hawkins E, Kirchner B (2011) Pnicogen bonds: a new molecular linker? Chem Eur J 17(22):6034–6038
Wang W, Ji B, Zhang Y (2009) Chalcogen bond: a sister noncovalent bond to halogen bond. J Phys Chem A 113:8132–8135
Cavallo G, Metrangolo P, Milani R, Pilati T, Priimagi A, Resnati G, Terraneo G (2016) The halogen bond. Chem Rev 116:2478–2601
Bauzá A, Frontera A (2015) Aerogen bonding interaction: a new supramolecular force? Angew Chem Int Ed 54:7340–7343
Cavallo G, Metrangolo P, Pilati T, Resnati G, Terraneo G (2014) Naming interactions from the electrophilic site. Cryst Growth Des 14:2697–2702
Legon AC (2017) Tetrel, pnictogen and chalcogen bonds identified in the gas phase before they had names: a systematic look at non-covalent interactions. Phys Chem Chem Phys 19(23):14884–14896
Legon AC, Walker NR (2018) What’s in a name? ‘Coinage-metal’ non-covalent bonds and their definition. Phys Chem Chem Phys 20:19332–19338
Cui J, Zhang X, Meng L, Li Q, Zeng Y (2019) Coinage metal dimers as the noncovalent interaction acceptors: study of the σ-lump interactions. Phys Chem Chem Phys 21:21152–21161
Zheng B, Liu Y, Wang Z, Zhou F, Jiao Y, Liu Y, Ding X, Li Q (2018) Comparison of halide donators based on pi···M (M=Cu, Ag, Au), pi···H and pi···halogen bonds. Theor Chem Acc 137:179
Moss RA, Doyle MP (2014) Contemporary carbene chemistry. Wiley, Hoboken
Alkorta I, Elguero J (1996) Carbenes and silylenes as hydrogen bond acceptors. J Phys Chem 100:19367–71930
Del Bene JE, Alkorta I, Elguero J (2017) Carbenes as electron-pair donors for P···C pnicogen bonds. ChemPhysChem 18(12):1597–1610
Alkorta I, Montero-Campillo MM, Elguero J (2017) Trapping CO2 by adduct formation with nitrogen heterocyclic carbenes (NHCs): a theoretical study. Chem-Eur J 23(44):10604–10609
Del Bene JE, Alkorta I, Elguero J (2017) Carbon–carbon bonding between nitrogen heterocyclic carbenes and CO2. J Phys Chem A 121(42):8136–8146
Li Q, Wang H, Liu Z, Li W, Sun J (2009) Ab initio study of lithium-bonded complexes with carbene as an electron donor. J Phys Chem A 113(51):14156–14160
Zhuo H, Li Q (2015) Novel pnicogen bonding interactions with silylene as an electron donor: covalency, unusual substituent effects and new mechanisms. Phys Chem Chem Phys 17(14):9153–9160
Liu M, Li Q, Li W, Cheng J (2017) Carbene tetrel-bonded complexes. Struct Chem 28(3):823–831
Chi Z, Dong W, Li Q, Yang X, Scheiner S, Liu S (2019) Carbene triel bonds between TrR3 (Tr = B, Al) and N-heterocyclic carbenes. Int J Quantum Chem 119(8):e25867
Lin H, Meng L, Li X, Zeng Y, Zhang X (2019) Comparison of pnicogen and tetrel bonds in complexes containing CX2 carbenes (X = F, Cl, Br, OH, OMe, NH2, and NMe2). New J Chem 43:15596–15604
Costa P, Fernandez-Oliva M, Sanchez-Garcia E, Sander W (2014) The highly reactive benzhydryl cation isolated and stabilized in water ice. J Am Chem Soc 136:15625–15630
Costa P, Sander W (2014) Hydrogen bonding switches the spin state of diphenylcarbene from triplet to singlet. Angew Chem Int Ed 53:5122–5125
Henkel S, Costa P, Klute L, Sokkar P, Fernandez-Oliva M, Thiel W, Sanchez-Garcia E, Sander W (2016) Switching the spin state of diphenylcarbene via halogen bonding. J Am Chem Soc 138(5):1689–1697
Ge Y, Lu Y, Xu Z, Liu H (2018) Controlling the spin state of diphenylcarbene via halogen bonding: a theoretical study. Int J Quantum Chem 118(15):e25616
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Ma R, Cheeseman JR, Scamani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, BloinoJ ZG, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian09. Gaussian Inc., Wallingford
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19(4):553–566
Bulat FA, Toro-Labbé A, Brinck T, Murray JS, Politzer P (2010) Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16(11):1679–1691
Keith TA (2015) Computational improvements for the theory of atoms in molecules. AIMAll (Version 15.09.27), TK Gristmill Software, Overland Park KS
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38
Scheiner S (2018) Steric crowding in tetrel bonds. J Phys Chem A 122:2550–2562
Scheiner S, Lu J (2018) Halogen, chalcogen, and pnicogen bonding involving hypervalent atoms. Chem Eur J 24:8167–8177
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132(18):6498–6506
Contreras-Garcia J, Johnson ER, Keinan S, Chaudret R, Piquemal JP, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7(3):625–632
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91(5):893–928
Becke A, Matta CF, Boyd RJ (2007) The quantum theory of atoms in molecules. Wiley, New York
Zeng Y, Zhang X, Li X, Meng L, Zheng S (2011) The role of molecular electrostatic potentials in the formation of a halogen bond in Furan···XY and Thiophene···XY complexes. ChemPhysChem 12:1080–1087
Rozas I, Alkorta I, Elguero J (2000) Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J Am Chem Soc 122:11154–11161
Grabowski SJ (2012) QTAIM characteristics of halogen bond and related interactions. J Phy Chem A 116:1838–1845
Cremer D, Kraka E (1984) Chemical bonds without bonding electron density—does the difference electron-density analysis suffice for a description of the chemical bond? Angew Chem Int Ed 23(23):627–628
Bone RGA, Bader RFW (1996) Identifying and analyzing intermolecular bonding interactions in van der Waals molecules. J Phys Chem 100(26):10892–10911
Politzer P, Murray JS, Clark T (2015) Mathematical modeling and physical reality in noncovalent interactions. J Mol Model 21:52
Zheng S, Hada M, Nakatsuji H (1996) Topology of density difference and force analysis. Theor Chim Acta 93:67–78
Li W, Zeng Y, Li X, Sun Z, Meng L (2016) Insight into the pseudo p-hole interactions in the M3H6(NCF)n (M = C, Si, Ge, Sn, Pb; n = 1, 2, 3) complexes. Phys Chem Chem Phys 18:24672–24680
Lu B, Zhang X, Meng L, Zeng Y (2017) Insight into π-hole interactions containing the inorganic heterocyclic compounds S2N2/SN2P2. J Mol Model 23:233
Code availability
N/A.
Funding
This work was supported by the National Natural Science Foundation of China (Contract no. 21973027 of Prof. Xiaoyan Li), Natural Science Foundation of Hebei Province (Contract no. B2020205002 of Prof. Xiaoyan Li), and the Foundation of Hebei Normal University (Contract no. L2018Z04 of Dr. Xueying Zhang).
Author information
Authors and Affiliations
Contributions
Chunhong Zhao: investigation and writing—original draft; Hui Lin: formal analysis and data curation; Aiting Shan: visualization; Shaofu Guo: writing—review and editing; Xiaoyan Li: methodology and supervision; Xueying Zhang: conceptualization and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Written informed consent for publication was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, C., Lin, H., Shan, A. et al. Theoretical study on the noncovalent interactions involving triplet diphenylcarbene. J Mol Model 27, 224 (2021). https://doi.org/10.1007/s00894-021-04838-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04838-6