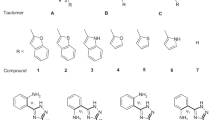
Abstract
Recent studies have identified N2,N4-bis(4-fluorophenethyl)-N6-(3-(dimethylamino)propyl)-1,3,5-triazine-2,4,6-triamine (1TZ(7,8,9)) as a potent, pure antagonist that inhibits thermosensory transient receptor potential vanilloid 1 channel (TRPV1) channel activity. This study provides theoretical data on the stability and acidity of the tautomers of this molecule. We show that this triazine can exist as three predominant tautomers (2TZ(5,7,8), 4TZ(3,7,9), 7TZ(1,8,9)). In the aqueous phase, equilibrium constants calculations show that only the tautomeric equilibria between 1TZ(7,8,9) and the three most stable triazines can be present which suggests that these three tautomeric equilibria would be the basis of 1TZ(7,8,9)’s biological activity.





Similar content being viewed by others
Data availability
N/A
References
Shao X, Zuo M, Zhao C et al (2020) Solubility of 2,4-diamino-6-(4-pyridyl)-1,3,5-triazine in tetrahydrofuran and alcohols between 288.15 K and 318.15 K. J Mol Liq 309:113170. https://doi.org/10.1016/j.molliq.2020.113170
Ni-Komatsu L, Leung JK, Williams D et al (2005) Triazine-based tyrosinase inhibitors identified by chemical genetic screening. Pigment Cell Res 18:447–453. https://doi.org/10.1111/j.1600-0749.2005.00273.x
Chen C, Chen Q, Kang J et al (2020) Hydrophilic triazine-based dendron for copper and lead adsorption in aqueous systems: performance and mechanism. J Mol Liq 298:112031. https://doi.org/10.1016/j.molliq.2019.112031
Ghasemian M, Kakanejadifard A, Azarbani F et al (2014) The triazine-based azo–azomethine dyes; spectroscopy, solvatochromism and biological properties of 2,2′-((2,2′-(6-methoxy-1,3,5-triazine-2,4-diyl) bis(oxy)bis(2,1-phenylene))bis(azan-1-yl-1-ylidene)bis(methan-1-yl-1-ylidene))bis(4-phenyldiazenyl)phenol. J Mol Liq 195:35–39. https://doi.org/10.1016/j.molliq.2014.01.011
Baryshnikov GV, Bondarchuk SV, Minaeva VA et al (2017) Solvatochromic effect in absorption and emission spectra of star-shaped bipolar derivatives of 1,3,5-triazine and carbazole. A time-dependent density functional study. J Mol Model 23:55. https://doi.org/10.1007/s00894-017-3234-y
Martins EPS, de Lima EO, Martins FT et al (2019) Synthesis, spectroscopic characterization, DFT studies, and preliminary antimicrobial evaluation of new antimony(III) and bismuth(III) complexes derived from 1,3,5-triazine. J Mol Struct 1183:373–383. https://doi.org/10.1016/j.molstruc.2019.01.096
Tomašić T, Kovač A, Klebe G et al (2012) Virtual screening for potential inhibitors of bacterial MurC and MurD ligases. J Mol Model 18:1063–1072. https://doi.org/10.1007/s00894-011-1139-8
Chen M, Hu D, Li X et al (2015) Antiviral activity and interaction mechanisms study of novel glucopyranoside derivatives. Bioorg Med Chem Lett 25:3840–3844. https://doi.org/10.1016/j.bmcl.2015.07.068
Marín-Ocampo L, Veloza LA, Abonia R, Sepúlveda-Arias JC (2019) Anti-inflammatory activity of triazine derivatives: a systematic review. Eur J Med Chem 162:435–447. https://doi.org/10.1016/j.ejmech.2018.11.027
Bhat HR, Singh UP, Thakur A et al (2015) Synthesis, antimalarial activity and molecular docking of hybrid 4-aminoquinoline-1,3,5-triazine derivatives. Exp Parasitol 157:59–67. https://doi.org/10.1016/j.exppara.2015.06.016
Cascioferro S, Parrino B, Spanò V et al (2017) 1,3,5-Triazines: a promising scaffold for anticancer drugs development. Eur J Med Chem 142:523–549. https://doi.org/10.1016/j.ejmech.2017.09.035
Zhang C-L, Liu Y-X, Zhang X-M et al (2018) Synthesis, characterization, DNA/HSA interactions and in vitro cytotoxic activities of two novel water-soluble copper(II) complexes with 1,3,5-triazine derivative ligand and amino acids. Mater Sci Eng C 91:414–425. https://doi.org/10.1016/j.msec.2018.05.065
Ismail AM, Abou El Maaty WM, Jean-Claude BJ, Mostafa SI (2019) Synthesis, characterization and anticancer activity of new Zn(II) and MoO22+ complexes of 2-amino-4,6-mercaptotriazine. Inorg Chem Commun 106:217–223. https://doi.org/10.1016/j.inoche.2019.04.024
Kala RS, Tharmaraj P, Sheela CD (2014) Synthesis, spectral studies, NLO, and biological studies on metal(II) complexes of s-triazine-based ligand. Synth React Inorg Metal-Orga Nano-Metal Chem 44:1487–1496. https://doi.org/10.1080/15533174.2013.818018
Demeshko S, Leibeling G, Dechert S, Meyer F (2004) 1,3,5-Triazine-based tricopper(II) complexes: structure and magnetic properties of threefold-symmetric building blocks. Dalton Trans:3782–3787. https://doi.org/10.1039/B407598F
Burton SJ, Vivian Stead C, Ansell RJ, Lowe CR (1996) An artificial redox coenzyme based on a triazine dye template. Enzym Microb Technol 18:570–580. https://doi.org/10.1016/0141-0229(96)00136-6
Puthiaraj P, Lee Y-R, Zhang S, Ahn W-S (2016) Triazine-based covalent organic polymers: design, synthesis and applications in heterogeneous catalysis. J Mater Chem A 4:16288–16311. https://doi.org/10.1039/C6TA06089G
Mellor IR, Ogilvie J, Pluteanu F et al (2004) Methoctramine analogues inhibit responses to capsaicin and protons in rat dorsal root ganglion neurons. Eur J Pharmacol 505:37–50. https://doi.org/10.1016/j.ejphar.2004.10.005
Kitaguchi T, Swartz KJ (2005) An inhibitor of TRPV1 channels isolated from funnel web spider venom. Biochemistry 44:15544–15549. https://doi.org/10.1021/bi051494l
Vidal-Mosquera M, Fernández-Carvajal A, Moure A et al (2011) Triazine-based vanilloid 1 receptor open channel blockers: design, synthesis, evaluation, and SAR analysis. J Med Chem 54:7441–7452. https://doi.org/10.1021/jm200981s
De Petrocellis L, Schiano Moriello A, Byun JS et al (2015) Inhibitory effect of positively charged triazine antagonists of prokineticin receptors on the transient receptor vanilloid type-1 (TRPV1) channel. Pharmacol Res 99:362–369. https://doi.org/10.1016/j.phrs.2015.07.009
Harris VH, Smith CL, Jonathan Cummins W et al (2003) The effect of Tautomeric constant on the specificity of nucleotide incorporation during DNA replication: support for the rare tautomer hypothesis of substitution mutagenesis. J Mol Biol 326:1389–1401. https://doi.org/10.1016/S0022-2836(03)00051-2
Katritzky AR, Hall CD, El-Gendy BE-DM, Draghici B (2010) Tautomerism in drug discovery. J Comput Aided Mol Des 24:475–484. https://doi.org/10.1007/s10822-010-9359-z
Martin YC (2009) Let’s not forget tautomers. J Comput Aided Mol Des 23:693. https://doi.org/10.1007/s10822-009-9303-2
Kasetti Y, Bharatam PV (2012) Tautomerism in drugs with benzimidazole carbamate moiety: an electronic structure analysis. Theor Chem Accounts 131:1160. https://doi.org/10.1007/s00214-012-1160-8
Bartolini B, Corniello C, Sella A et al (2003) The enol tautomer of indole-3-pyruvic acid as a biological switch in stress responses. In: Allegri G, Costa CVL, Ragazzi E et al (eds) Developments in tryptophan and serotonin metabolism. Springer US, Boston, MA, pp 601–608
Srinivas K, Sitha S, Sridhar B et al (2006) Tautomerism of bis(2,4-benzyloxy)-6-(5H)-one-1,3,5-triazine: a combined crystallographic and quantum-chemical investigation. Struct Chem 17:561–568. https://doi.org/10.1007/s11224-006-9058-5
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627. https://doi.org/10.1021/j100096a001
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J Chem Phys 72:5639–5648. https://doi.org/10.1063/1.438980
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654. https://doi.org/10.1063/1.438955
Anandan K, Kolandaivel P, Kumaresan R (2004) Ab initio and DFT studies on tautomerism of indazole in gaseous and aqueous phases. J Mol Struct THEOCHEM 686:83–89. https://doi.org/10.1016/j.theochem.2004.08.014
Karthika M, Senthilkumar L, Kanakaraju R (2014) Hydrogen-bond interactions in hydrated 6-selenoguanine tautomers: a theoretical study. Struct Chem 25:197–213. https://doi.org/10.1007/s11224-013-0239-8
Senthilkumar L, Kolandaivel P (2003) Post Hartree–Fock and density functional theory studies on tautomerism of 6-thioxanthine in gas phase and in solution. J Mol Struct THEOCHEM 638:69–78. https://doi.org/10.1016/S0166-1280(03)00546-3
Umadevi V, Mano Priya A, Senthilkumar L (2015) DFT study on the tautomerism of organic linker 1H-imidazole-4,5-tetrazole (HIT). Comput Theor Chem 1068:149–159. https://doi.org/10.1016/j.comptc.2015.06.022
Semmeq A, Ouaskit S, Monari A, Badawi M (2019) Ionization and fragmentation of uracil upon microhydration. Phys Chem Chem Phys 21:4810–4821. https://doi.org/10.1039/C8CP07452F
Semmeq A, Monari A, Badawi M, Ouaskit S (2019) Ab initio study of the stepwise versus concerted fragmentation pathways in microhydrated thymine radical Cations. Chem Eur J 25:15525–15534. https://doi.org/10.1002/chem.201902462
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani J, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski, VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, in, Gaussian, Inc., Wallingford
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001. https://doi.org/10.1021/jp9716997
Mezey PG, Ladik JJ (1979) A non-empirical molecular orbital study on the relative stabilities of adenine and guanine tautomers. Theoret Chim Acta 52:129–145. https://doi.org/10.1007/BF00634788
Mezey PG, Ladik JJ, Barry M (1979) Non-empirical SCF MO studies on the protonation of biopolymer constituents. Theoret Chim Acta 54:251–258. https://doi.org/10.1007/BF00578344
Elguero J (2013) Tautomerism. In: Maloy S, Hughes K (eds) Brenner’s encyclopedia of genetics2nd edn. Academic Press, San Diego, pp 18–22
Remko M (2003) Theoretical study of molecular structure and gas-phase acidity of some biologically active sulfonamides. J Phys Chem A 107:720–725. https://doi.org/10.1021/jp026980m
Rogstad KN, Jang YH, Sowers LC, Goddard WA (2003) First principles calculations of the pKa values and tautomers of isoguanine and xanthine. Chem Res Toxicol 16:1455–1462. https://doi.org/10.1021/tx034068e
Jang YH, Goddard WA, Noyes KT et al (2003) pKa values of guanine in water: density functional theory calculations combined with Poisson−Boltzmann continuum−solvation model. J Phys Chem B 107:344–357. https://doi.org/10.1021/jp020774x
Acknowledgements
We thank the PMMS (Pôle Messin de Modélisation et de Simulation) and the LPCT high-performance computing resources for providing us with computer time. We also thank the colleagues of Constitution and Reaction of Matter Laboratory of University Felix Houphouët Boigny for their useful advices.
Code availability
N/A
Author information
Authors and Affiliations
Contributions
Lucie Affoue Bede: performing the actual study, writing the first draft.
Alain Kouassi Koffi: writing the first draft.
Fred-Lawson Ekozias Digre Beke: writing the first draft.
Abderrahmane Semmeq: performing the actual study, Correcting the draft to final form.
Michael Badawi: Reviewing, assessing, and correcting the draft to final form.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bede, L.A., Koffi, A.K., Beke, FL.E.D. et al. Investigation of tautomerism of 1,3,5-triazine derivative, stability, and acidity of its tautomers from density functional theory. J Mol Model 27, 147 (2021). https://doi.org/10.1007/s00894-021-04774-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04774-5