Abstract
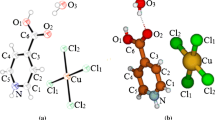
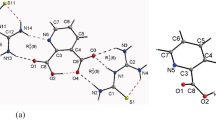
The detailed illustrations of the structures, elastic properties, and Raman and IR vibration modes for [Na(H2O)(N5)]·2H2O (a) and [Mg(H2O)6(N5)2]·4H2O (b) have been presented in this investigation by using the first-principles method based on the density functional theory. Our results indicate that the active centers of both two types of the energetic metal pentazolate hydrates appear on the cyclo-N5. The bonding character of N atoms in the cyclo-N5 is shown to be covalent, and the cyclo-N5 ring can be considered as an anion. Based on the analysis of elastic properties, we conclude that complex a is easier to deform than b, and both complexes are mechanically stable. From the calculated Raman and IR vibration modes, the vibration in the region of 960–1206 cm−1 (for a) and 985–1208 cm−1 (for b) is determined by basically mixing the cyclo-N5 stretching and deformation modes. The vibrational modes of a and b in their highest frequency zones are both related to the stretching of the O–H bonds.









Similar content being viewed by others
References
Christe KO (2007) Recent advances in the chemistry of N5+, N5− and high-oxygen compounds. Propellants Explos Pyrotech 32:194–204
Hirshberg B, Gerber RB, Krylov AI (2014) Calculations predict a stable molecular crystal of N8. Nat Chem. 6:52–56
Christe KO (2017) Polynitrogen chemistry enters the ring. Science 355:351
Xu YG, Wang Q, Shen C, Lin QH, Wang PC, Lu M (2017) A series of energetic metal pentazolate hydrates. Nature 549:78–81
Curtius T, Darapsky A, Müller E (1915) Die sogenannten Pentazol-Verbindungen von J. Lifschitz. Eur J Inorg Chem 48:1614–1634
Clusius K, Hürzeler H (1954) Reaktionen mit 15N. XII. Mechanismus der Phenylazidbildung bei der Diazoreaktion. Helvetica Chimica Acta 37:798–804
Huisgen R, Ugi I (1956) Zur Lösung eines klassischen Problems der organischen Stickstoff-Chemie. Angew Chem. 68:705–706
Huisgen R, Ugi I, Pentazole I (1957) Die Lösung Eines Klassischen Problems der Organischen Stickstoffchemie. Chemische Berichte 90:2914–2927
Witanowski M, Stefaniak L, Januszewski H, Bahadur K (1975) Nitrogen-14 nmr evidence for the pentazole ring structure. J Cryst Mol Struct 5:137–140
Müller R, Wallis JD, von Philipsborn W (1985) Direct structural proof for the pentazole ring system in solution by 15N-NMR spectroscopy. Angew Chem Int Ed. 24:513–515
Wallis JD, Dunitz JD (1983) An all-nitrogen aromatic ring system: structural study of 4-dimethyl-aminophenylpentazole. J Chem Soc Chem Commun:910–911
Vij A, Pavlovich JG, Wilson WW, Vij V, Christe KO (2002) Experimental detection of the pentaazacyclopentadienide (pentazolate) anion, cyclo-N5−. Angew Chem. 114:3177–3180
Nguyen MT (2003) Polynitrogen compounds 1. Structure and stability of N4 and N5 systems. Coord Chem Rev 244:93–113
Schroer T, Haiges R, Schneider S, Christe KO (2005) The race for the first generation of the pentazolate anion in solution is far from over. Chem Commun.:1607–1609
Butler RN, Stephens JC, Burke LA (2003) First generation of pentazole (HN5, pentazolic acid), the final azole, and a zinc pentazolate salt in solution: a new N-dearylation of 1-(p-methoxyphenyl) pyrazoles, a 2-(p-methoxyphenyl) tetrazole and application of the methodology to 1-(p-methoxyphenyl) pentazole. Chem Commun:1016–1017
Bazanov B, Geiger U, Carmieli R, Grinstein D, Welner S, Haas Y (2016) Detection of Cyclo-N5− in THF solution. Angew Chem 128:13427–13429
Zhang C, Sun CG, Hu BC, Yu CM, Lu M (2017) Synthesis and characterization of the pentazolate anion cyclo-N5— in (N5)6(H3O)3(NH4)4Cl. Science 355:374–376
Zhang C, Yang C, Hu BC, Yu CM, Zheng ZS, Sun CG (2017) A symmetric co(N5)2(H2O)4·4H2O high-nitrogen compound formed by cobalt (II) Cation trapping of a Cyclo-N5− anion. Angew Chem Int Ed. 56:4512–4514
Yang C, Zhang C, Zheng Z, Jiang C, Luo J, Du Y, Hu B, Sun C, Christe KO (2018) Synthesis and characterization of cyclo-pentazolate salts of NH4+, NH3OH+, N2H5+, C(NH2)3+, and N (CH3)4+. J Am Chem Soc 140:16488–16494
Clark SJ, Segall MD, Pickard CJ, Hasnip PJ, Probert MIJ, Refson K, Payne MC (2005) First principles methods using CASTEP. Zeitschrift für Kristallographie-Crystalline Materials 220:567–570
Rappe AM, Rabe KM, Kaxiras E, Joannopoulos JD (1990) Optimized pseudopotentials. Phys Rev B 41:1227
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Appalakondaiah S, Vaitheeswaran G, Lebègue S (2013) A DFT study on structural, vibrational properties, and quasiparticle band structure of solid nitromethane. J Chem Phys 138:184705
Vaitheeswaran G, Babu KR, Yedukondalu N, Appalakondaiah S (2014) Structural properties of solid energetic materials: a van der Waals density functional study. Curr Sci 106:1219
Birch F (1978) Finite strain isotherm and velocities for single-crystal and polycrystalline NaCl at high pressures and 300 K. J Geophys Res Solid Earth 83:1257–1268
Setyawan W, Curtarolo S (2010) High-throughput electronic band structure calculations: Challenges and tools. Comput Mater Sci 49:299
Bondarchuk SV, Minaev BF (2017) Two isomeric solid carbon nitrides with 1: 1 stoichiometry which exhibit strong mechanical anisotropy. New J Chem 41:13140
Segall MD, Shah R, Pickard CJ, Payne MC (1996) Population analysis of plane-wave electronic structure calculations of bulk materials. Phys Rev B 54:16317
Segall MD, Pickard CJ, Shah R, Payne MC (1996) Population analysis in plane wave electronic structure calculations. Mol. Phys. 89:571–577
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44:129–138
Mulliken RS (1955) Electronic population analysis on LCAO–MO molecular wave functions. I. J Chem Phys 23:1833–1840
Silva AM, Silva BP, Sales FAM, Freire VN (2012) Optical absorption and DFT calculations in L-aspartic acid anhydrous crystals: charge carrier effective masses point to semiconducting behavior. Phys Rev B 86:195201
Böhlke T, Brüggemann C (2001) Graphical representation of the generalized Hooke’s law. Tech Mech. 21:145–158
Mouhat F, Coudert FX (2014) Necessary and sufficient elastic stability conditions in various crystal systems. Phys Rev B 90:224104
Chung DH, Buessem WR (1968) The Voigt-Reuss-Hill (VRH) approximation and the elastic moduli of polycrystalline ZnO, TiO2 (rutile), and α-Al2O3. J Appl Phys 39:2777–2782
Liu QJ, Liu ZT (2014) Structural, elastic, and mechanical properties of germanium dioxide from first-principles calculations. Mater Sci Semicond Process 27:765–776
Pugh SF (1954) XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos Mag 45:823–843
Ranganathan SI, Ostoja-Starzewski M (2008) Universal elastic anisotropy index. Phys Rev Lett 101:055504
Feng J, Xiao B, Zhou R, Pan W (2013) Anisotropy in elasticity and thermal conductivity of monazite-type REPO4 (RE= La, Ce, Nd, Sm, Eu and Gd) from first-principles calculations. Acta Mater. 61:7364–7383
Chen XQ, Niu HY, Li DZ, Li YY (2011) Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 19:1275–1281
Tian Y, Xu B, Zhao Z (2012) Microscopic theory of hardness and design of novel superhard crystals. Int J Refract Met Hard Mater 33:93–106
Patterson JD, Bailey BC (2007) Solid-state physics: introduction to the theory. Springer Science & Business Media, pp 12–23
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, H., Zhu, SH., Gan, YD. et al. The Raman and IR vibration modes of metal pentazolate hydrates [Na(H2O)(N5)]·2H2O and [Mg(H2O)6(N5)2]·4H2O. J Mol Model 26, 84 (2020). https://doi.org/10.1007/s00894-020-4345-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-4345-4