Abstract
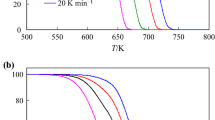
The fractional derivative concept to treat non-isothermal solid state thermal decomposition was employed in this work. Simulated data were compared with the exact solutions for the method validation. Calculated fractional kinetics data for four heating rates were initially considered and the Kissinger-Akahira-Sunose (KAS) method demonstrate that, although the activation energy is not retrieved, it can be useful to determine a single or multistep process. Experimental thermal decomposition data of lumefantrine heated at 5, 10 ,15, and 20 oC min− 1 were fitted for a single-step process. The kinetic parameters were retrieved for integer and fractional kinetics, considering some ideal and general models. Application of the KAS method to these data demonstrated an activation energy dependent on the conversion rate, indicating a multistep process. Five data subintervals were fitted separately using the general model with variable derivative order. It was found a process that occours with integer order derivative until α = 0.3 and fractional order for α > 0.3 with combination of simultaneous reactions, since the parameters do not correspond to any ideal model. The determined activation energies showed the same increasing behavior observed in the KAS approach. The results for multistep process presented an error 102 times smaller if compared with the best result, considering a single-step process. Therefore, the fractional kinetic model presents a powerful extension to the usual thermal data analysis.









Similar content being viewed by others
References
Lewis GN (1905) Zersetzung von silberoxyd durch autokatalyse. Z Phys Chem 52:310–326. https://doi.org/10.1515/zpch-1905-5219
Centnerszwer M, Bruzs B (1925) The thermal decomposition of silver carbonate. J Phys Chem 29:733–737. https://doi.org/10.1021/j150252a008
Avrami M (1039) Kinetics of phase change. I General theory. J Phys Chem 7:1103–1112. https://doi.org/10.1063/1.1750380
Prout EG, Tompkins FC (1944) The thermal decomposition of potassium permanganate. Trans Faraday Soc 40:488–498. https://doi.org/10.1039/TF9444000488
Erofe’ev BV (1946) Generalized equation of chemical kinetics and its application in reactions involving solids. Compt Rend Acad Sci USSR 52:511–514
Zanatta ER, Reinehr TO, Awadallak JA, et al. (2016) Kinetic studies of thermal decomposition of sugarcane bagasse and cassava bagasse. J Therm Anal Calorim 125:437–445. https://doi.org/10.1007/s10973-016-5378-x
Kissinger HE (1956) Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand 57:217–221
Flynn J, Wall LA (1966) A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B Polym Lett 4:323–328. https://doi.org/10.1002/pol.1966.110040504
Ozawa T (1965) A new method of analyzing thermogravimetric. Bull Chem Soc Jpn 38:1881–1886. https://doi.org/10.1246/bcsj.38.1881
Akahira T (1971) Trans. Joint convention of four electrical institutes. Res Rep Chiba Inst Technol 16:22–31
Vyazovkin S, Wight CA (1997) Isothermal and nonisothermal reaction kinetics in solids: in search of ways toward consensus. J Phys Chem A 101:8279–8284. https://doi.org/10.1021/jp971889h
Sebastião RCO, Braga JP, Yoshida MI (2003) Artificial neural network applied to solid state thermal decomposition. J Therm Anal Calorim 74:811–818. https://doi.org/10.1023/B:JTAN.0000011013.80148.46
Sebastião RCO, Braga JP, Yoshida MI (2004) Competition between kinetic models in thermal decomposition: analysis by artificial neural network. Thermochim Acta 412:107–111. https://doi.org/10.1016/j.tca.2003.09.009
Pomerantsev AL, Kutsenova AV, Rodionova OY (2017) Kinetic analysis of non-isothermal solid-state reactions: multi-stage modeling without assumptions in the reaction mechanism. Phys Chem Chem Phys 19:3606–3615. https://doi.org/10.1039/C6CP07529K
Podlubny I (1998) Fractional differential equations: an introduction to fractional derivatives, fractional differential equations to methods of their solution and some of their applications. Elsevier, New York
Miller KS, Ross B (1993) An introduction to the fractional calculus and fractional differential equations. Wiley-Interscience, New York
Tarasov VE (2013) Review of some promising fractional physical models. Int J Mod Phys B 27:1330005–1–1330005-32. https://doi.org/10.1142/S0217979213300053
Lemes NHT, dos Santos JPC, Braga JP (2016) A generalized Mittag-Leffler function to describe nonexponential chemical effects. Appl Math Model 40:7971–7976. https://doi.org/10.1016/j.apm.2016.04.021
Gibson M (2016) Pharmaceutical preformulation and formulation: a practical guide from candidate drug selection to commercial dosage form. CRC Press, New York
BRASIL (2019) Agência Nacional De Vigilância Sanitária. Resolução de Diretoria Colegiada, RDC n 301, de 21 de agosto de 2019. Dispõe sobre as Diretrizes Gerais de Boas práticas de fabricação de Medicamentos. Diá,rio Oficial da União, 22 ago
Ministério da Saúde (2010) Guia prático de Tratamento da malária no Brasil. In: Fontes CJF, Santelli ACFS, Silva CJM, Tauil PL, Ladislau JLB (eds) Ministério da saúde, Brasília, Brasil
Cai J, Liu R (2009) Kinetic analysis of solid-state reactions: a general empirical kinetic model. Ind Eng Chem Res 48:3249–3253. https://doi.org/10.1021/ie8018615
Karaca A, Sarikaya MZ (2014) On the k-Riemann-Liouville fractional integral and applications. Int J Stat Math 1:33–43
Dimitrov Y (2013) Numerical approximations for fractional differential equations. arXiv:1311.3935
Freitas-Marques MB, Araújo B, Mussel WN, Yoshida MI, Fernandes C, Sebastião RCO (2019) Kinetics of lumefantrine thermal decomposition employing isoconversional models and artificial neural network. J Braz Chem Soc
D’Errico J (2012) Bound constrained optimization using fminsearch. https://www.mathworks.com/matlabcentral/fileexchange/8277-fminsearchbnd-fminsearchcon. Accessed 10 jun 2019
Lagarias JC, Reeds JA, Wright MH, Wright PE (1998) Convergence properties of the Nelder-Mead simplex method in low dimensions. SIAM J Optimiz 9:112–147. https://doi.org/10.1137/S1052623496303470
World Health Organization The International Pharmacopoeia; 8 Ed.; World Health Organization, v. 1
Cavalheiro ET, Ionashiro M, Breviglieri ST, Marino G, Chierice GO (1995) A influência de fatores experimentais nos resultados de análises termogravimétricas. Quí,mica Nova 18:305–308
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda L A, Popescu C, Sbirrazzuoli N. (2011) ICTAC Kinetics Committee Recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19. https://doi.org/10.1016/j.tca.2011.03.034
Funding
We would like to thank CNPq for financial support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection XX - Brazilian Symposium of Theoretical Chemistry (SBQT2019).
Rights and permissions
About this article
Cite this article
Carvalho, F.S., Braga, J.P., Marques, M.B.F. et al. Fractional kinetics on thermal analysis: application to lumefantrine thermal decomposition. J Mol Model 26, 170 (2020). https://doi.org/10.1007/s00894-020-04360-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04360-1