Abstract
The evolution of structural properties, thermodynamics and averaged (dynamic) total hardness values as a function of the composition of binary water–organic solvents, was rationalized in view of the intermolecular interactions. The organic solvents considered were ethanol, acetonitrile, and isopropanol at 0.25, 0.5, 0.75, and 1 mass fractions, and the results were obtained using molecular dynamics simulations. The site-to-site radial distribution functions reveal a well-defined peak for the first coordination shell in all solvents. A characteristic peak of the second coordination shell exists in aqueous mixtures of acetonitrile, whereas in the water–alcohol solvents, a second peak develops with the increase in alcohol content. From the computed coordination numbers, averaged hydrogen bonds and their lifetimes, we found that water mixed with acetonitrile largely preserves its structural features and promotes the acetonitrile structuring. Both the water and alcohol structures in their mixtures are disturbed and form hydrogen bonds between molecules of different kinds. The dynamic hardness values are obtained as the average over the total hardness values of 1200 snapshots per solvent type, extracted from the equilibrium dynamics. The dynamic hardness profile has a non-linear evolution with the liquid compositions, similarly to the thermodynamic properties of these non-ideal solvents.

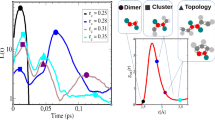
Computed dynamic total hardness, as a function of the cosolvent mass fraction for water–ethanol (EtOH), water–isopropanol (2PrOH) and water–acetonitrile (AN)






Similar content being viewed by others
References
Saiz L, Padro JA, Guardia E (1999) Dynamics and hydrogen bonding in liquid ethanol. Mol Phys 97(7):897–905
Chang S et al (2012) Solvent polarity and internal stresses control the swelling behaviour of green wood during dehydration in organic solution. Bioresources 7(2):2418–2430
Smith MD et al (2016) Cosolvent pretreatment in cellulosic biofuel production: effect of tetrahydrofuran-water on lignin structure and dynamics. Green Chem 18(5):1268–1277
Smith MD et al (2016) Molecular driving forces behind the tetrahydrofuran–water miscibility gap. J Phys Chem B 120(4):740–747
Li M-F, Yang S, Sun R-C (2016) Recent advances in alcohol and organic acid fractionation of lignocellulosic biomass. Bioresour Technol 200:971–980
Bossu J, Le Moigne N, Corn S, Trens P, Di Renzo F (2018) Sorption of water–ethanol mixtures by poplar wood: swelling and viscoelastic behaviour. Wood Sci Technol 52(4):987–1008. https://doi.org/10.1007/s00226-018-1022-1
Jawad ZA et al (2016) The role of solvent mixture, acetic acid and water in the formation of CA membrane for CO2/N2 separation. Procedia Eng 148:327–332
Pires RM et al (2007) Viscosity and density of water + ethyl acetate + ethanol mixtures at 298.15 and 318.15 K and atmospheric pressure. J Chem Eng Data 52(4):1240–1245
Hurle RL, Easteal AJ, Woolf LA (1985) Self-diffusion in monohydric alcohols under pressure. Methanol, methan (2 H) ol and ethanol. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases 81(3):769–779
Pang F-M et al (2007) Densities and viscosities of aqueous solutions of 1-propanol and 2-propanol at temperatures from 293.15 K to 333.15 K. J Mol Liq 136(1):71–78
Grande MdC, Bianchi HL, Marschoff CM (2004) On the density of pure acetonitrile. Anales de la Asociación Química Argentina 92:109–114
Cunningham GP, Vidulich GA, Kay RL (1967) Several properties of acetonitrile-water, acetonitrile-methanol, and ethylene carbonate-water systems. J Chem Eng Data 12(3):336–337
Lide DR (2004) CRC handbook of chemistry and physics. 12J204
Peeters D, Huyskens P (1993) Endothermicity or exothermicity of water/alcohol mixtures. J Mol Struct 300(Supplement C):539–550
Stokes RH (1987) Excess partial molar enthalpies for (acetonitrile + water) from 278 to 318 K. J Chem Thermodyn 19(9):977–983
Wakisaka A et al (1998) Non-ideality of binary mixtures water–methanol and water–acetonitrile from the viewpoint of clustering structure. J Chem Soc Faraday Trans 94(3):369–374
Shaker-Gaafar N et al (1993) p,T-dependence of self-diffusion in liquid ethanol and the propanols. Ber Bunsenges Phys Chem 97(6):805–811
Hurle RL, Woolf LA (1982) Self-diffusion in liquid acetonitrile under pressure. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases 78(7):2233–2238
Li Z et al (2017) Molecular dynamics simulation of self-diffusion coefficients for several alkanols. Russ J Phys Chem A 91(7):1260–1269
Akerlof G (1932) Dielectric constants of some organic solvent-water mixtures at various temperatures. J Am Chem Soc 54(11):4125–4139
Wyman J (1931) The dielectric constant of mixtures of ethyl alcohol and water from-5 to 40. J Am Chem Soc 53(9):3292–3301
Venables DS, Schmuttenmaer CA (1998) Far-infrared spectra and associated dynamics in acetonitrile–water mixtures measured with femtosecond THz pulse spectroscopy. J Chem Phys 108(12):4935–4944
Rivelino R et al (2005) Electronic polarization in liquid acetonitrile: a sequential Monte Carlo/quantum mechanics investigation. Chem Phys Lett 407(1):13–17
Jorgensen WL, Briggs JM (1988) Monte Carlo simulations of liquid acetonitrile with a three-site model. Mol Phys 63(4):547–558
Evans M (1983) Molecular dynamics and structure of liquid acetonitrile—a review and computer simulation. J Mol Liq 25(3):149–175
Oldiges C et al (2002) MD calculated structural properties of clusters in liquid acetonitrile/water mixtures with various contents of acetonitrile. J Phys Chem A 106(31):7147–7154
Nikitin AM, Lyubartsev AP (2007) New six-site acetonitrile model for simulations of liquid acetonitrile and its aqueous mixtures. J Comput Chem 28(12):2020–2026
Lindquist BA, Haws RT, Corcelli SA (2008) Optimized quantum mechanics/molecular mechanics strategies for nitrile vibrational probes: acetonitrile and para-tolunitrile in water and tetrahydrofuran. J Phys Chem B 112(44):13991–14001
Sato T, Chiba A, Nozaki R (2000) Hydrophobic hydration and molecular association in methanol–water mixtures studied by microwave dielectric analysis. J Chem Phys 112(6):2924–2932
Sato T, Chiba A, Nozaki R (1999) Dynamical aspects of mixing schemes in ethanol–water mixtures in terms of the excess partial molar activation free energy, enthalpy, and entropy of the dielectric relaxation process. J Chem Phys 110(5):2508–2521
Sato T, Chiba A, Nozaki R (2000) Composition-dependent dynamical structures of 1-propanol–water mixtures determined by dynamical dielectric properties. J Chem Phys 113(21):9748–9758
Sato T, Buchner R (2003) Dielectric relaxation spectroscopy of 2-propanol–water mixtures. J Chem Phys 118(10):4606–4613
Matsugami M et al (2016) Hydrogen bonding in ethanol–water and trifluoroethanol–water mixtures studied by NMR and molecular dynamics simulation. J Mol Liq 217:3–11
Pozar M et al (2016) Micro-heterogeneity versus clustering in binary mixtures of ethanol with water or alkanes. Phys Chem Chem Phys 18(34):23971–23979
Petong P, Pottel R, Kaatze U (2000) Water−ethanol mixtures at different compositions and temperatures. A dieletric relaxation study. J Phys Chem A 104(32):7420–7428
Saiz L, Padró JA, Guàrdia E (1997) Structure and dynamics of liquid ethanol. J Phys Chem B 101(1):78–86
Lam RK, Smith JW, Saykally RJ (2016) Communication: hydrogen bonding interactions in water-alcohol mixtures from X-ray absorption spectroscopy. J Chem Phys 144(19):191103
Finneran IA et al (2015) Hydrogen bonding in the ethanol-water dimer. Phys Chem Chem Phys 17(37):24210–24214
Alavi S et al (2010) Hydrogen-bonding alcohol-water interactions in binary ethanol, 1-propanol, and 2-propanol+methane structure II clathrate hydrates. J Chem Phys 133(7):074505
Pothoczki S, Pusztai L (2017) Intermolecular orientations in liquid acetonitrile: new insights based on diffraction measurements and all-atom simulations. J Mol Liq 225:160–166
Robertson RE, Sugamori SE (1972) The hydrolysis of t-butyl chloride in aquo-organic mixtures: heat capacity of activation and solvent structure. Can J Chem 50(9):1353–1360
Moreau C, Douhéret G (1975) Thermodynamic behavior of water-acetonitrile mixtures excess volumes and viscosities. Thermochim Acta 13(4):385–392
Damewood JR, Kumpf RA (1987) Hydration of polar organic molecules: the interaction of acetonitrile with water. J Phys Chem 91(12):3449–3452
Kovacs H, Laaksonen A (1991) Molecular dynamics simulation and NMR study of water-acetonitrile mixtures. J Am Chem Soc 113(15):5596–5605
Bertie JE, Lan Z (1997) Liquid water−acetonitrile mixtures at 25 °C: the hydrogen-bonded structure studied through infrared absolute integrated absorption intensities. J Phys Chem B 101(20):4111–4119
Takamuku T et al (1998) Liquid structure of acetonitrile−water mixtures by X-ray diffraction and infrared spectroscopy. J Phys Chem B 102(44):8880–8888
Bakó I, Megyes T, Pálinkás G (2005) Structural investigation of water–acetonitrile mixtures: an ab initio, molecular dynamics and X-ray diffraction study. Chem Phys 316(1):235–244
Rowlen KL, Harris JM (1991) Raman spectroscopic study of solvation structure in acetonitrile/water mixtures. Anal Chem 63(10):964–969
Abraham MJ et al (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25
Bekker H, Berendsen HJC, Dijkstra EJ, Achterop S, Vondrumen R, Vanderspoel D, Sijbers A, Keegstra H, Renardus MKR (1993) GROMACS: a parallel computer for molecular dynamics simulations. In: DeGroot RA, Nadrchal J (eds) Physics computing '92. World Scientific, Songapore, pp 252–256
Berendsen HJC, van der Spoel D, van Drunen R (1995) GROMACS: a message-passing parallel molecular dynamics implementation. Comput Phys Commun 91(1):43–56
Van Der Spoel D et al (2005) GROMACS: fast, flexible, and free. J Comput Chem 26(16):1701–1718
Páll S et al Tackling exascale software challenges in molecular dynamics simulations with GROMACS. In: Markidis S, Laure E (eds) Solving software challenges for exascale: international conference on exascale applications and software, EASC 2014, Stockholm, Sweden, April 2–3, 2014, Revised Selected Papers 2015, Springer, Cham, pp 3–27
Guvench O, Greene SN, Kamath G, Brady JW, Venable RM, Pastor RW, Mackerell AD Jr (2008) Additive empirical force field for hexopyranose monosaccharides. J Comput Chem 29:2543–2564
Guvench O, Hatcher ER, Venable RM, Pastor RW, Mackerell AD (2009) CHARMM additive all-atom force field for glycosidic linkages between hexopyranoses. J Chem Theory Comput 5:2353–2370
Jorgensen WL et al (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79(2):926–935
Dick TJ, Madura JD (2005) Chapter 5, A Review of the TIP4P, TIP4P-Ew, TIP5P, and TIP5P-E Water Models. in Annual Reports in Computational Chemistry. Elsevier, p 59–74
Mark P, Nilsson L (2001) Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J Phys Chem A 105(43):9954–9960
van der Spoel D, van Maaren PJ, Caleman C (2012) GROMACS molecule & liquid database. Bioinformatics 28(5):752–753
Nosé S, Klein ML (1983) Constant pressure molecular dynamics for molecular systems. Mol Phys 50(5):1055–1076
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52(12):7182–7190
Luzar A, Chandler D (1996) Hydrogen-bond kinetics in liquid water. Nature 379:55
Luzar A (2000) Resolving the hydrogen bond dynamics conundrum. J Chem Phys 113(23):10663–10675
van der Spoel D et al (2006) Thermodynamics of hydrogen bonding in hydrophilic and hydrophobic media. J Phys Chem B 110(9):4393–4398
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85(22):3533–3539
Pearson RG (1997) Chemical hardness. Wiley Online Library
Parr RG et al (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68(8):3801–3807
Nalewajski RF (1993) The hardness based molecular charge sensitivities and their use in the theory of chemical reactivity. In: Sen KD (ed) Chemical hardness. Springer, Berlin, pp 115–186
Neshev N, Mineva TZ (1996) The role of interelectronic interaction in transition metal oxide catalysts. In: Russo N, Salahub DR (eds) MMetal-ligand interactions: structure and reactivity. Springer, Dordrecht, pp 361–405
Senet P (1996) Nonlinear electronic responses, Fukui functions and hardnesses as functionals of the ground-state electronic density. J Chem Phys 105(15):6471–6489
Senet P (1997) Kohn-sham orbital formulation of the chemical electronic responses, including the hardness. J Chem Phys 107(7):2516–2524
Chandrakumar KRS, Pal S (2002) A systematic study on the reactivity of Lewis acid−base complexes through the local hard−soft acid−base principle. J Phys Chem A 106(48):11775–11781
Chattaraj PK, Roy DR (2007) Update 1 of: electrophilicity index. Chem Rev 107(9):PR46–PR74
Mineva T, Heine T (2004) Efficient computation of density-functional orbitally resolved reactivity indices. J Phys Chem A 108(50):11086–11091
Mineva T et al (1997) Density functional potential-energy hypersurface and reactivity indices in the isomerization of X3H+ (X = O, S, Se, Te). J Chem Soc Faraday Trans 93(18):3309–3312
Mineva T, Sicilia E, Russo N (1998) Density-functional approach to hardness evaluation and its use in the study of the maximum hardness principle. J Am Chem Soc 120(35):9053–9058
Mineva T et al (1999) Gas-phase properties and Fukui indices of sulfine (CH2SO). Potential energy surface and maximum hardness principle for its protonation process. A density functional study. Theor Chem Accounts 101(6):388–395
Madjarova G et al (2005) Selectivity descriptors for the Michael addition reaction as obtained from density functional based approaches. J Phys Chem A 109(2):387–393
Mineva T (2006) Selectivity study from the density functional local reactivity indices. J Mol Struct THEOCHEM 762(1):79–86
Mineva T, Thomas H (2006) Orbital hardness tensors from hydrogen through xenon from Kohn–Sham perturbed orbitals. Int J Quantum Chem 106(6):1396–1405
Ohno K (1967) Molecular orbital calculations of Φ electron systems. In: Löwdin PO (ed) Advances in quantum chemistry. Academic, New York, pp 239–322
Bjelkmar P et al (2010) Implementation of the CHARMM force field in GROMACS: analysis of protein stability effects from correction maps, virtual interaction sites, and water models. J Chem Theory Comput 6(2):459–466
Anisimov VM et al (2007) Polarizable empirical force field for the primary and secondary alcohol series based on the classical Drude model. J Chem Theory Comput 3(6):1927–1946
Guàrdia E et al (2001) Comparison of different three-site interaction potentials for liquid acetonitrile. Mol Simul 26(4):287–306
Soper AK, Phillips MG (1986) A new determination of the structure of water at 25°C. Chem Phys 107(1):47–60
Jerie K et al (2005) Structure of aqueous solutions of acetonitrile investigated by acoustic and positron annihilation measurements. Acta Physica Polonica-Series A General Physics 107(5):826–831
Xu H, Stern HA, Berne BJ (2002) Can water polarizability be ignored in hydrogen bond kinetics? J Phys Chem B 106(8):2054–2060
Soper AK, Bruni F, Ricci MA (1997) Site–site pair correlation functions of water from 25 to 400 °C: revised analysis of new and old diffraction data. J Chem Phys 106(1):247–254
Noskov SY, Lamoureux G, Roux B (2005) Molecular dynamics study of hydration in ethanol−water mixtures using a polarizable force field. J Phys Chem B 109(14):6705–6713
Salamatova E et al (2017) Hydrogen bond and lifetime dynamics in diluted alcohols. Phys Chem Chem Phys 19(41):27960–27967
Acknowledgments
This work was granted access to the HPC resources of [CCRT/CINES/IDRIS] under the allocation 2017 [×2017087369] made by GENCI (Grand Equipement National de Calcul Intensif] and was partially funded through the ENSCM SINCHEM EMJD PhD grant. SINCHEM is a Joint Doctorate programme selected under the Erasmus Mundus Action 1 Programme (FPA 2013-0037).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection International Conference on Systems and Processes in Physics, Chemistry and Biology (ICSPPCB-2018) in honor of Professor Pratim K. Chattaraj on his sixtieth birthday
Electronic supplementary material
ESM 1
(PDF 964 kb)
Rights and permissions
About this article
Cite this article
Aguilera-Segura, S.M., Di Renzo, F. & Mineva, T. Structures, intermolecular interactions, and chemical hardness of binary water–organic solvents: a molecular dynamics study. J Mol Model 24, 292 (2018). https://doi.org/10.1007/s00894-018-3817-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3817-2