Abstract
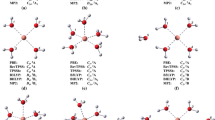
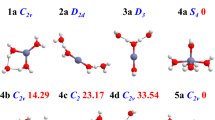
The potential energy surface of [Cu(H2O)n]2+ clusters with n = 12, 16, and 18 was explored by using a modified version of the simulated annealing method. Such exploration was carried out by using the PM7 semiempirical method to obtain around 100,000 isomers, which provide candidates to be optimized with PBE0-D3, M06-2X, and BHLYP exchange-correlation functionals coupled with the 6–311++G** basis set. These methods based on the Kohn-Sham approach delivered isomers with coordination numbers of 4, 5, and 6. The analysis used to obtain coordination numbers was based on geometrical parameters and the quantum theory of atoms in molecules (QTAIM) approach. Our methodology found only one isomer with fourfold coordination and its probabilities to appear in these clusters are quite small for high temperatures. The procedure used in this article predicts important populations of fivefold and sixfold coordination clusters, in fact, the fivefold coordination dominates for PBE0-D3 and BHLYP methods, although the sixfold coordination starts to be important when the number of water molecules is increased. The nature of axial and equatorial contacts is discussed in the context of the QTAIM and the noncovalent interaction index (NCI), which gives a clear classification of such orientations. Also, these methods suggest a partial covalent interaction between the Cu2+ and water molecules in both positions; equatorial and axial.






Similar content being viewed by others
References
Jones CJ, Thornback J (2007) Medicinal applications of coordination chemistry. Royal Society of Chemistry, Cambridge
Strausak D, Mercer JF, Dieter HH, Stremmel W, Multhaup G (2001) Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull 55:175–185
Mercer SW, Wang J, Burke R (2017) In vivo modeling of the pathogenic effect of copper transporter mutations that cause Menkes and Wilson diseases, motor neuropathy, and susceptibility to Alzheimer’s disease. J Bio Chem 292:4113–4122
Bowron DT, Amboage M, Boada R, Freeman A, Hayamab S, Díaz-Moreno S (2013) The hydration structure of Cu2+: more tetrahedral than octahedral? RSC Adv 3:17803–17812
Frank P, Benfatto M, Szilagyi RK, D’Angelo P, Della Longa S, Hodgson KO (2005) The solution structure of [cu(aq)]2+ and its implications for rack-induced bonding in blue copper protein active sites. Inorg Chem 44:1922–1933
Garcia J, Benfatto M, Natoli C, Bianconi A, Fontaine A, Tolentino H (1989) The quantitative Jahn-teller distortion of the Cu2+ site in aqueous solution by xanes spectroscopy. Chem Phys 132:295–302
Galván-García EA, Agacino-Valdés E, Franco-Pérez M, Gómez-Balderas R (2017) [Cu(H2O)n]2+(n=1-6) complexes in solution phase: a DFT hierarchical study. Theor Chem Accounts 136:29
Schwenk CF, Rode BM (2004) Influence of electron correlation effects on the solvation of Cu2+. J Am Chem Soc 126:12786–12787
Amira S, Spångberg D, Hermansson K (2005) Distorted five-fold coordination of Cu2+ (aq) from a car-parrinello molecular dynamics simulation. Phys Chem Chem Phys 7:2874–2880
Pasquarello A, Petri I, Salmon PS, Parisel O, Car R, Tóth É, Powell DH, Fischer HE, Helm L, Merbach AE (2001) First solvation shell of the Cu (II) aqua ion: evidence fivefold coordination. Science 291:856–859
Li X, Tu Y, Tian H, Ågren H (2010) Computer simulations of aqua metal ions for accurate reproduction of hydration free energies and structures. J Chem Phys 132:104505
Bryantsev VS, Diallo MS, Van Duin AC, Goddard IIIWA (2008) Hydratation of copper (II): new insights from density functional theory and the COSMO solvatation model. J Phys Chem A 112:9104–9112
Rios-Font R, Sodupe M, Rodríguez-Santiago L, Taylor PR (2010) The role of exact exchange in the description of Cu2+((H2O)n (n= 1− 6)) complexes by means of DFT methods. J Phys Chem A 114:10857–10863
Salmon P, Howells W, Mills R (1987) The dynamics of water molecules in ionic solution. II. Quasi-elastic neutron scattering and tracer diffusion studies of the proton and ion dynamics in concentrated Ni2+, Cu2+ and Nd3+ aqueous solutions. J Phys C Solid State Phys 20:5727
Powell DH, Helm L, Merbach AE (1991) 17O nuclear magnetic resonance in aqueous solutions of Cu2+: the combined effect of Jahn-teller inversion and solvent exchange on relaxation rates. J Chem Phys 95:9258–9265
Kirkpatrick S, Gelatt CD, Vecchi MP (1983) Optimization by simulated annealing. Sci 220:671–680
Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E (1953). J Chem Phys 21:1087–1092
Aarts E, Laarhoven H (1987) Simulated annealing: theory and applications. Springer, New York
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University, New York
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang W (2010) Revealing non-covalent interactions. J Am Chem Soc 132:6498–6506
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal JP, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632
Pérez JF, Hadad C, Restrepo A (2008) Structural studies of the water tetramer. Int J Quantum Chem 108:1653–1659
Pérez JF, Florez E, Hadad CZ, Fuentealba P, Restrepo A (2008) Stochastic search of the quantum conformational space of small lithium and bimetallic lithium-sodium clusters. J Phys Chem A 112:5749–5755
Stewart JJ (2013) Optimization of parameters for semi-empirical methods vi: more modifications to the nddo approximations and re-optimization of parameters. J Mol Model 19:1–32
Stewart JJP (2008) MOPAC2009, Stewart computational chemistry, Colorado Springs, CO. Available at OpenMOPAC. net. Accessed 13 May 2016
Zhao Y, Truhlar DG (2006) A new local density functional for main group thermochemistry, transition metal bonding, thermochemical kinetics, and non-covalent interactions. J Chem Phys 125:194101
Zhao Y, Truhlar DG (2011) Applications and validations of the Minnesota density functionals. Chem Phys Lett 502:1–13
Rassolov VA, Pople JA, Ratner MA, Windus TL (1998) 6-31g* basis set for atoms K through Zn. J Chem Phys 109:1223–1229
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77:3654–3665
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170
Andersson MP, Uvdal P (2005) New scale factors for harmonic vibrational frequencies using the b3lyp density functional method with the triple-ζ basis set 6-311+g(d, p). J Phys Chem A 109:2937–2941
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465
Valiev M, Bylaska EJ, Govind N, Kowalski K, Straatsma TP, van Dam HJJ, Wang D, Nieplocha J, Apra E, Windus TL, de Jong WA (2010) NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations. Comput Phys Commun 181:1477–1489
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, Revision D.01. Gaussian Inc, Wallingford
Benfatto M, D’ Angelo P, Della Longa S, Pavel N (2002) Evidence of distorted fivefold coordination of the Cu2+ aqua ion from an x-ray absorption spectroscopy quantitative analysis. Phys Rev B 65:174205
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98:1372–1377
Hernández-Esparza R, Mejía-Chica SM, Zapata-Escobar AD, Guevara-García A, Martínez-Melchor A, Hernández-Pérez JM, Vargas R, Garza J (2014) Grid based algorithm to search critical points, in the electron density, accelerated by graphics processing units. J Comp Chem 35:2272–2278
Kumar PSV, Raghavendra V, Subramanian V (2016) Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding. J Chem Sci 128:1527–1536
Ash T, Debnath T, Banu T, Das AK (2016) Exploration of binding interactions of Cu2+ with D-penicillamine and its O-and se-analogues in both gas and aqueous phases: a theoretical approach. J Phys Chem B 120:3467–3478
Bryantsev VS, Diallo MS, Goddard IIIWA (2009) Computational study of copper (II) complexation and hydrolysis in aqueous solutions using mixed cluster/continuum models. J Phys Chem A 113:9559–9567
Duncombe BJ, Duale K, Buchanan-Smith A, Stace AJ (2007) The solvation of Cu2+ with gas-phase clusters of water and ammonia. J Phys Chem A 111:5158–5165
Berces A, Nukada T, Margl P, Ziegler T (1999) Solvation of Cu2+ in water and ammonia. Insight from static and dynamical density functional theory. J Phys Chem A 103:9693–9701
Persson I (2010) Hydrated metal ions in aqueous solution: how regular are their structures? Pure Appl Chem 82:1901–1917
Noyes RM (1962) Thermodynamics of ion hydration as a measure of effective dielectric properties of water. J Am Chem Soc 84:513–522
Acknowledgments
The authors thank the facilities provided by the Laboratorio de Supercómputo y Visualización en Paralelo at the Universidad Autónoma Metropolitana-Iztapalapa. A. Monjaraz-Rodríguez and M. Rodriguez-Bautista thank CONACYT, México, for the scholarships 286378 and 283261, respectively.
This article was written to recognize the contributions of Professor Pratim Kumar Chattaraj to Density Functional Theory and celebrate his 60th anniversary.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection International Conference on Systems and Processes in Physics, Chemistry and Biology (ICSPPCB-2018) in honor of Professor Pratim K. Chattaraj on his sixtieth birthday
Electronic supplementary material
ESM 1
(PDF 2734 kb)
Rights and permissions
About this article
Cite this article
Monjaraz-Rodríguez, A., Rodriguez-Bautista, M., Garza, J. et al. Coordination numbers in hydrated Cu(II) ions. J Mol Model 24, 187 (2018). https://doi.org/10.1007/s00894-018-3725-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3725-5