Abstract
Two novel amide-based receptors were synthesized under microwave irradiation. Their chemical structures were confirmed by IR, 1H NMR, 13C NMR, and elemental analysis. The binding properties of these amide-based receptors to various anions (H2PO4 −, HSO4 −, C6H5CO2 −, CH3CO2 −, ClO4 −, F−, Cl−, and Br−) were examined by UV titration in THF at 20 °C. The results indicated that the receptors form 1:1 complexes with anions and they have the strongest affinity for fluoride (F−) among the anions considered. Molecular dynamics calculations by AMBER and quantum mechanical calculations performed at the B3LYP and M062X levels of theory using the 6-31 + g(d,p) basis set provided models for the complexation mode between the receptors and anions and yielded binding energies for the complexes.

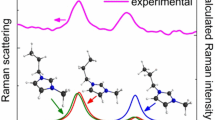
The computed interaction mode of tripodals (1a and 1b) with fluoride.











Similar content being viewed by others
References
Beer PD, Shade M (1997) Solvent dependent anion selectivity exhibited by neutral ferrocenoyl receptors. Chem Commun 24:2377–2378
Shao J, Yu X, Lin H, Lin H (2008) Colorimetric recognizing of biologically important anions based on anion-induced tautomerism of the sensor. J Mol Recognit 21:425–430
Bondy CR, Loeb SJ (2003) Amide based receptors for anions. Coord Chem Rev 240:77–99
Christianson DW, Lipscomb WN (1989) Carboxypeptidase A. Acc Chem Res 22:62–69
Berg JM (1995) Zinc finger domains: from predictions to design. Acc Chem Res 28:14–19
Beer PD, Gale PA (2001) Anion recognition and sensing: the state of the art and future perspectives. Angew Chem Int Ed 40:486–516
Wenzel M, Hiscock JR, Gale PA (2010) Anion receptor chemistry: highlights from 2010. Chem Rev 41:480–520
Dutta R, Ghos P (2012) Encapsulation of fluoride/chloride in the C3v-symmetric cleft of a pentafluorophenyl-functionalized cyanuric acid platform based tripodal amide: solid and solution-state anion-binding studies. Eur J Inorg Chem 3456–3462
Saha S, Akhuli B, Ravikumar I, Lakshminarayanan PS, Ghosh P (2014) Recognition of fluoride in fluorophenyl attached tripodal amide receptors: structural evidence of solvent capped encapsulation of anion in a C3v-symmetric tripodal cleft. Cryst Eng Comm 16:4796–4804
Wang HB, Wisner JA, Jennings MC (2010) Anion receptors containing thiazine-1,1-dioxide heterocycles as hydrogen bond donors. Beilstein J Org Chem 6:50
Lanigan RM, Starkov P, Sheppard TD (2013) Direct synthesis of amides from carboxylic acids and amines using B(OCH2CF3)3. J Org Chem 78:4512–4523
Lidstrom P, Tierney JP, Wathey B, Westman J (2001) Microwave assisted organic synthesis—a review. Tetrahedron 57:9225–9283
Tierney JP, Lidstrom P (2005) Microwave assisted organic synthesis. Blackwell, Oxford
Hayes BL (2002) Microwave synthesis: chemistry at the speed of light. CEM, Matthews
Loupy A (2002) Microwaves in organic synthesis. Wiley-VCH, Weinheim
Varma RS (2002) Advances in green chemistry: chemical syntheses using microwave irradiation. AstraZeneca Research Foundation India, Bangalore
Bogdal D (2005) Microwave-assisted organic synthesis: one hundred reaction procedures. Elsevier, Oxford
Kappe CO (2004) Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed 43:6250–6284
Zhao Z, Liu M, Liu F, Wang X (2014) Microwave-assisted synthesis and anion recognition of novel bile acid-based molecular tweezers using 4-nitrobenzoylhydrazines and chiral unsymmetrical urea units as arms. J Southwest Univ Natl: Nat Sci Ed 40:195–202
Perreux L, Loupy A, Volatron F (2002) Solvent-free preparation of amides from acids and primary amines under microwave irradiation. Tetrahedron 58:2155–2162
Chen Y, Zhao Z, Liu X, Shi Z (2011) A facile and efficient approach to the synthesis of novel chiral molecular tweezers based on deoxycholic acid under microwave irradiation. Lett Org Chem 8:210–215
Ozturk G, Colak M, Togrul M (2010) Amide-based tripodal receptors for selective anion recognition. J Incl Phenom Macrocycl Chem 60:49–54
Li W, Zhao Z, Liu X, Wang X (2010) Solvent-free synthesis of aryl ethers promoted by tetrabutylammonium fluoride. J Chem Res 34:399–402
Wong MW, Ghosh T, Maiya BG (2004) Fluoride ion receptors based on dipyrrolyl derivatives bearing electron-withdrawing groups: synthesis, optical and electrochemical sensing, and computational studies. J Phys Chem A 108:11249–11259
Singh NJ, Jun EJ, Chellappan K, Thangadurai D, Chandran RP, Hwang IC, Yoon J, Kim KS (2007) Quinoxaline-imidazolium receptors for unique sensing of pyrophosphate and acetate by charge transfer. Org Lett 9:485–488
Ruangpornvisuti V (2004) Recognition of carboxylate and dicarboxylates by azophenol-thiourea derivatives: a theoretical host–guest investigation. J Mol Struct THEOCHEM 686:47–55
Mondal CK, Lee JY (2006) Understanding of molecular functions: computational approaches. J Theor Comput Chem 5:857–869
Jose DA, Singh A, Das A, Ganguly B (2007) A density functional study towards the preferential binding of anions to urea and thiourea. Tetrahedron Lett 48:3695–3698
Rakrai W, Morakot N, Keawwangchai S, Kaewtong C, Wanno B, Ruangpornvisuti V (2011) A theoretical investigation on structures of tripodal thiourea derivatives and their anion recognition. Struct Chem 22:839–847
Xie H, Wong MW (2012) Computational design of thiourea-based cyclophane sensors for small anions. Aust J Chem 65:303–313
Mong MW, Xie H, Kwa ST (2013) Anion recognition by azophenol thiourea-based chromogenic sensors: a combined DFT and molecular dynamics investigation. J Mol Model 19(1):205–213
Zhao Y, Schultz NE, Truhlar DG (2005) Exchange-correlation functional with broad accuracy for metallic and nonmetallic compounds, kinetics, and noncovalent interactions. J Chem Phys 123:161103
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125:194101
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Kocakaya SO, Turgut Y, Pirinççioğlu N (2015) Enantiomeric discrimination of chiral organic salts by chiral aza-15-crown-5 ether with C 1 symmetry: experimental and theoretical approaches. J Mol Model 55:1–13
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision E.01. Gaussian Inc., Wallingford
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Lee DH, Lee HY, Lee KH, Hong JI (2001) Selective anion sensing based on a dual-chromophore approach. Chem Commun 1188–1189
Lee DH, Lee KH, Hong JI (2001) An azophenol-based chromogenic anion sensor. Org Lett. 3:5–8
Amendola V, Esteban-Gomez D, Fabbrizzi L, Licchelli M (2006) What anions do to N–H containing receptors. Acc Chem Res 39:343–353
Acknowledgements
We are grateful for the financial support from The Scientific and Technological Research Council of Turkey (TUBITAK) with project no. 109 T787 and from Dicle University Research Council (DÜBAP) with project 09-FF-67.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOC 77434 kb)
Rights and permissions
About this article
Cite this article
Öztürk, G., Subari, S., Şeker, S. et al. A facile synthesis of amide-based receptors under microwave conditions: investigation of their anion recognition properties by experimental and computational tools. J Mol Model 23, 249 (2017). https://doi.org/10.1007/s00894-017-3390-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3390-0