Abstract
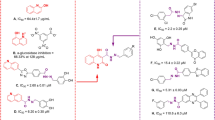
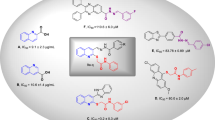
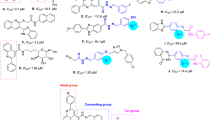
Apigenin is an important flavonoids due to its antidiabetic bioactivity. It was reported experimentally that the 7-substituent derivative of apigenin has higher biological activity than 4′- and 5-substituted derivatives while introducing sole carboxyalkyl group -(CH2)7COOH into the parent structure. Molecular docking studies indicated that the other two derivatives have lower binding affinities than the 7-substituent derivative (-7.52 kcal mol−1), which is considered to be a better inhibitor than the parent molecule. Almost all of the carbon atoms and oxygen atoms are coplaner for all three molecules in solution phase, however, all carboxyalkyl groups bend inside into the parent molecules in the active site, and the jagged geometries of the carbon chains are destroyed correspondingly. In addition, most of the electron densities of the chemical bonds for all molecules are decreased, especially the 7-substituent derivative. In contrast, most of the Laplacian values for three molecules are increased in the active site, which suggests that the charge densities at the bond critical point (bcp) are much more depleted than the solution phase. Dipole moments of derivatives are all increased in the active site, suggesting strong intermolecular interactions. After interacting with the S. cerevisiae α-glucosidase, only the 7-substituent derivative has the lowest energy gap ΔE HOMO-LUMO, which indicates the lowest stability and the highest inhibition activity.

Probing the influence of carboxyalkyl groups on the molecular flexibility and the charge density of apigenin derivatives












Similar content being viewed by others
References
Israili HZ (2011) Advances in the treatment of type 2 diabetes mellitus. Am J Ther 18:117–152. doi:10.1097/MJT.0b013e3181afbf51
Bischoff H (1995) The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med 18(4):303–311
Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P (2012) Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med 156:218–231. doi:10.7326/0003-4819-156-3-201202070-00011
Moorthy NS, Ramos MJ, Fernandes PA (2012) Studies on α-glucosidase inhibitors development: Magic molecules for the treatment of carbohydrate mediated diseases. Mini-Rev Med Chem 12:713–720. doi:10.2174/138955712801264837
Maltarollo VG, Homem-de-Mello P, Honório KM (2010) Theoretical study on the molecular and electronic properties of some substances used for diabetes mellitus treatment. J Mol Model 16(4):799–804. doi:10.1007/s00894-009-0627-6
Kalra S (2014) Alpha glucosidase inhibitors. J Pak Med Assoc 64:474–476
Etxeberria U, de la Garza AL, Campión J, Martínez JA, Milagro FI (2012) Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin Ther Targets 16:269–297. doi:10.1517/14728222.2012.664134
Mokale SN, Palkar AD, Dube PN, Sakle NS, Miniyar PB (2016) Design, synthesis and in vivo screening of some novel quinazoline analogs as anti-hyperlipidemic and hypoglycemic agents. Bioorg Med Chem Lett 26:272–276. doi:10.1016/j.bmcl.2015.12.037
Sakkiah S, Thangapandian S, Lee KW (2012) Pharmacophore modeling, molecular docking, and molecular dynamics simulation approaches for identifying new lead compounds for inhibiting aldose reductase 2. J Mol Model 18(7):3267–3282. doi:10.1007/s00894-011-1247-5
Kumar S, Narwal S, Kumar V, Prakash O (2011) α-glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev 5(9):19–29. doi:10.4103/0973-7847.79096
Bibi S, Sakata K (2016) Current status of computer-aided drug design for type 2 diabetes. Curr Comput Aided Drug Des. doi:10.2174/1573409912666160426120709
de Assis TM, Gajo GC, de Assis LC, Garcia LS, Silva DR, Ramalho TC, da Cunha EF (2016) QSAR models guided by molecular dynamics applied to human glucokinase activators. Chem Biol Drug Des 87:455–466. doi:10.1111/cbdd.12683
Yan J, Zhang G, Pan J, Wang Y (2014) α-Glucosidase inhibition by luteolin: kinetics, interaction and molecular docking. Int J Biol Macromol 64:213–223. doi:10.1016/j.ijbiomac.2013.12.007
Chen AY, Chen YC (2013) A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem 138:2099–2107. doi:10.1016/j.foodchem.2012.11.139
Jin Q, Yang J, Ma L, Cai J, Li J (2015) Comparison of polyphenol profile and inhibitory activities against oxidation and α-glucosidase in mulberry (genus Morus) cultivars from China. J Food Sci 80(11):C2440–2451. doi:10.1111/1750-3841.13099
Na B, Nguyen PH, Zhao BT, Vo QH, Min BS, Woo MH (2016) Protein tyrosine phosphatase 1B (PTP1B) inhibitory activity and glucosidase inhibitory activity of compounds isolated from Agrimonia pilosa. Pharm Biol 54(3):474–480. doi:10.3109/13880209.2015.1048372
Li H, Song F, Xing J, Tsao R, Liu Z, Liu S (2009) Screening and structural characterization of alpha-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MS(n) and SORI-CID FTICR MS. J Am Soc Mass Spectrom 20(8):1496–1503. doi:10.1016/j.jasms.2009.04.003
Zhang J, Liu D, Huang Y, Gao Y, Qian S (2012) Biopharmaceutics classification and intestinal absorption study of apigenin. Int J Pharm 436(1-2):311–317. doi:10.1016/j.ijpharm.2012.07.002
Zhang L, Zuo Z, Lin G (2007) Intestinal and hepatic glucuronidation of flavonoids. Mol Pharm 4(6):833–845. doi:10.1021/mp700077z
Sroka Z, Żbikowska B, Hładyszowski J (2015) The antiradical activity of some selected flavones and flavonols. Experimental and quantum mechanical study. J Mol Model 12:307. doi:10.1007/s00894-015-2848-1
Kim MK, Park KS, Lee C, Park HR, Choo H, Chong Y (2010) Enhanced stability and intracellular accumulation of quercetin by protection of the chemically or metabolically susceptible hydroxyl groups with a pivaloxymethyl (POM) promoiety. J Med Chem 53(24):8597–8607. doi:10.1021/jm101252m
Wen X, Walle T (2006) Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos 34(10):1786–1792. doi:10.1124/dmd.106.011122
Su ZR, Fan SY, Shi WG, Zhong BH (2015) Discovery of xanthine oxidase inhibitors and/or α-glucosidase inhibitors by carboxyalkyl derivatization based on the flavonoid of apigenin. Bioorg Med Chem Lett 5(14):2778–2781. doi:10.1016/j.bmcl.2015.05.016
Raghavachari K, Saha A (2015) Accurate Composite and Fragment-Based Quantum Chemical Models for Large Molecules. Chem Rev 115(12):5643–5677. doi:10.1021/cr500606e
Liu J, Zhu T, Wang X, He X, Zhang JZ (2015) Quantum fragment based ab initio molecular dynamics for proteins. J Chem Theory Comput 11(12):5897–5905. doi:10.1021/acs.jctc.5b00558
da Silva Gonçalves A, França TC, Caetano MS, Ramalho TC (2014) Reactivation steps by 2-PAM of tabun-inhibited human acetylcholinesterase: reducing the computational cost in hybrid QM/MM methods. J Biomol Struct Dyn 32:301–307. doi:10.1080/07391102.2013.765361
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford, U.K
Guisasola EEB, Gutierrez LJ, Salcedo RE, Garibotto FM, Andujar SA, Enriz RD, Rodriguez AM (2016) Conformational transition of A beta(42) inhibited by a mimetic peptide. A molecular modeling study using QM/MM calculations and QTAIM analysis. Comput Theor Chem 1080:56–65. doi:10.1016/j.comptc.2016.02.002
de Ruyck J, Brysbaert G, Blossey R, Lensink MF (2016) Lensink, Molecular docking as a popular tool in drug design, an in silico travel. Adv Appl Bioinform Chem 9:1–11. doi:10.2147/AABC.S105289
Singh RN, Baboo V, Rawat P, Kumar A, Verma (2012) Molecular structure, spectral studies, intra and intermolecular interactions analyses in a novel ethyl 4-[3-(2-chloro-phenyl)-acryloyl] -3,5-dimethyl-1H-pyrrole-2-carboxylate and its dimer: a combined DFT and AIM approach. Spectrochim Acta A Mol Biomol Spectrosc 94:288–301. doi:10.1016/j.saa.2012.03.059
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JAJr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ,Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, revision E.01 (2009) Gaussian Inc., Wallingford
Espinoza-Hicks JC, Nápoles-Duarte JM, Nevárez-Moorillón GV, Camacho-Dávila A, Rodríguez-Valdez LM (2016) Synthesis, electronic, and spectral properties of novel geranylated chalcone derivatives: a theoretical and experimental study. J Mol Model 22(10):253. doi:10.1007/s00894-016-3114-x
Mohamed TA, Soliman UA, Shaaban IA, Zoghaib WM, Wilson LD (2015) Raman, infrared and NMR spectral analysis, normal coordinate analysis and theoretical calculations of 5-(methylthio)-1,3,4-thiadiazole- 2(3H)-thione and its thiol tautomer. Spectrochim Acta A Mol Biomol Spectrosc 150:339–349. doi:10.1016/j.saa.2015.05.039
Muñoz MA, Joseph-Nathan P (2009) DFT-GIAO 1H and 13C NMR prediction of chemical shifts for the configurational assignment of 6beta-hydroxyhyoscyamine diastereoisomers. Magn Reson Chem 47(7):578–584. doi:10.1002/mrc.2432
Tomasi J, Mennucci B (2005) Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105(8):2999–3093. doi:10.1021/cr9904009
Delgado A, Corni S, Pittalis S, Rozzi CA (2015) Modeling solvation effects in real-space and real-time within density functional approaches. J Chem Phys 143(14):144111. doi:10.1063/1.4932593
Lu T, Chen FW (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comp Chem 33:580–592. doi:10.1002/jcc.22885
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 16:2785–2791. doi:10.1002/jcc.21256
Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T (2009) The SWISS-MODEL repository and associated resources. Nucleic Acids Res 37:D387–D392. doi:10.1007/978-1-60761-977-2_3
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics 22:195–201. doi:10.1093/bioinformatics/bti770
van Bochove MA, Swart M, Bickelhaupt FM (2007) Nucleophilic substitution at phosphorus centers (SN2@P). ChemPhysChem 8:2452–2463. doi:10.1002/cphc.200700488
van Bochove MA, Bickelhaupt FM (2008) Nucleophilic substitution at C, Si and P: how solvation affects the shape of reaction profiles. Eur J Org Chem 4:649–654. doi:10.1002/ejoc.200700953
van Bochove MA, Swart M, Bickelhaupt FM (2009) Stepwise walden inversion in nucleophilic substitution at phosphorus. Phys Chem Chem Phys 11:259–267. doi:10.1039/b813152j
de Lima WEA, Pereira AF, de Castro AA, da Cunha EFF, Ramalho TC (2016) Flexibility in the molecular design of acetylcholinesterase reactivators: probing representative conformations by chemometric techniques and docking/QM calculations. Lett Drug Des Discov 13:360–371. doi:10.2174/1570180812666150918191550
Kessler J, Dračínský M, Bouř P (2013) Parallel variable selection of molecular dynamics clusters as a tool for calculation of spectroscopic properties. J Comp Chem 34:366–371. doi:10.1002/jcc.23143
Pearson K (1901) On lines and planes of closest fit to systems of points in space. Philos Mag 6:559–572
Imran S, Taha M, Ismail NH, Kashif SM, Rahim F, Jamil W, Hariono M, Yusuf M, Wahab H (2015) Synthesis of novel flavone hydrazones: in-vitro evaluation of α-glucosidase inhibition, QSAR analysis and docking studies. Eur J Med Chem 105:156–170. doi:10.1016/j.ejmech.2015.10.017
Qi YJ, Zhao YM, Lu HN, Wang XE, Jin NZ (2016) Comparative analysis of the bonding modes between two antidiabetic drugs with beta-glucosidases from different species. Indian J Pharm Sci 78(4):525–536. doi:10.4172/pharmaceutical-sciences.1000146
Yar M, Bajda M, Shahzadi L, Shahzad SA, Ahmed M, Ashraf M, Alam U, Khan IU, Khan AF (2014) Novel synthesis of dihydropyrimidines for a-glucosidase inhibition to treat type 2 diabetes: in vitro biological evaluation and in silico docking. Bioorg Chem 54:96–104. doi:10.1016/j.bioorg.2014.05.003
Devipriya B, Kumaradhas P (2013) Molecular flexibility and the electrostatic moments of curcumin and its derivatives in the active site of p300: a theoretical charge density study. Chem Biol Interact 204:153–156. doi:10.1016/j.cbi.2013.05.002
Yang HY, Murphy-Ullrich JE, Song YH (2016) Molecular insights for the role of key residues of calreticulin in its binding activities. Biophys J 110: p53a
Sudha A, Srinivasan P, Thamilarasan V, Sengottuvelan N (2016) Exploring the binding mechanism of 5-hydroxy-3′,4′,7-trimethoxyflavone with bovine serum albumin: Spectroscopic and computational approach, Spectrochim Acta. A Mol Biomol Spectrosc 157:170–181. doi:10.1016/j.saa.2015.12.028
Guo XC, He DY, Huang L, Liu LM, Liu LJ, Yang HJ (2012) Strain energy in enzyme-substrate binding: An energetic insight into the flexibility versus rigidity of enzyme active site. Comput Theor Chem 995:17–23. doi:10.1016/j.comptc.2012.06.017
Qi YJ, Zhao YM, Lu HN, Jin NZ (2016) Exploring molecular flexibility and the interactions of Quercetin derivatives in the active site of α-glucosidase using molecular docking and charge density analysis. Comput Theor Chem 1094:55–68. doi:10.1016/j.comptc.2016.09.004
Devipriya B, Kumaradhas P (2012) Probing the effect of intermolecular interaction and understanding the electrostatic moments of anacardic acid in the active site of p300 enzyme via DFT and charge density analysis. J Mol Graph Model 34:57–66. doi:10.1016/j.jmgm.2011.12.003
Azhagesan RP, Poomani K (2013) Exploring the conformation, charge density distribution and the electrostatic properties of galanthamine molecule in the active site of AChE using DFT and AIM theory. Int J Quant Chem 113:1200–1208. doi:10.1002/qua.24251
Cukrowski I, de Lange JH, Adeyinka AS, Mangondo P (2015) Evaluating common QTAIM and NCI interpretations of the electron density concentration through IQA interaction energies and 1D cross-sections of the electron and deformation density distributions. Comput Theor Chem 1053:60–76. doi:10.1016/j.comptc.2014.10.005
Kulkarni GU, Kumaradhas P, Rao CNR (1998) Charge density study of the polymorphs of p-nitrophenol. Chem Mater 10:3498–3505
Ren FD, Cao DL, Shi WJ, Gao HF (2016) A theoretical prediction of the relationships between the impact sensitivity and electrostatic potential in strained cyclic explosive and application to H-bonded complex of nitrocyclohydrocarbon. J Mol Model 22:97. doi:10.1007/s00894-016-2967-3
Selvaraju K, Jothi M, Kumaradhas P (2012) Understanding the charge density distribution and the electrostatic properties of hexadecane molecular nanowire under electric field using DFT and AIM theory. Comput Theor Chem 992:9–17. doi:10.1016/j.comptc.2012.04.019
Wawer I, Zielinska A (1997) 13C-CP-MAS-NMR studies of flavonoids. I. Solid-state conformation of quercetin, quercetin 5′-sulphonic acid and some simple polyphenols. Solid State Nucl Magn Reson 10:33–38
Balachandran V, Karpagam V, Revathi B, Kavimani M, Ilango G (2015) Conformational stability, spectroscopic and computational studies, HOMO-LUMO, NBO, ESP analysis, thermodynamic parameters of natural bioactive compound with anticancer potential of 2-(hydroxymethyl)anthraquinone. Spectrochim Acta A Mol Biomol Spectrosc 150:631–640. doi:10.1016/j.saa.2015.06.007
Elmoussaoui S, Korek M (2015) Electronic structure with dipole moment calculation of the low-lying electronic states of ZnBr molecule. Comput Theor Chem 1068:42–46. doi:10.1016/j.comptc.2015.06.015
Targema M, Obi-Egbedi NO, Adeoye MD (2013) Molecular structure and solvent effects on the dipole moments and polarizabilities of some aniline derivatives. Comput Theor Chem 1012:47–53. doi:10.1016/j.comptc.2013.02.020
Panicker CY, Varghese HT, Narayana B, Divya K, Sarojini BK, War JA, Van Alsenoy C, Fun HK (2015) FT-IR, NBO, HOMO–LUMO, MEP analysis and molecular docking study of Methyl N-({[2-(2-methoxyacetamido)-4-(phenylsulfanyl)phenyl]amino}[(methoxycarbonyl) imino] methyl) carbamate. Spectrochim Acta A Mol Biomol Spectrosc 148:29–42. doi:10.1016/j.saa.2015.03.064
Sambathkumar K, Jeyavijayan S, Arivazhagan M (2015) Electronic structure investigations of 4-aminophthal hydrazide by UV-visible, NMR spectral studies and HOMO-LUMO analysis by ab initio and DFT calculations. Spectrochim Acta A Mol Biomol Spectrosc 147:124–138. doi:10.1016/j.saa.2015.03.012
Khan KM, Qurban S, Salar U, Taha M, Hussain S, Perveen S, Hameed A, Ismail NH, Riaz M, Wadood A (2016) Synthesis, in vitro α-glucosidase inhibitory activity and molecular docking studies of new thiazole derivatives. Bioorg Chem 68:245–258. doi:10.1016/j.bioorg.2016.08.010
Wang G, Peng Z, Wang J, Li J, Li X (2016) Synthesis, biological evaluation and molecular docking study of N-arylbenzo[d]oxazol-2-amines as potential α-glucosidase inhibitors. Bioorg Med Chem 24(21):5374–5379
Sun H, Zhang Y, Ding W, Zhao X, Song X, Wang D, Li Y, Han K, Yang Y, Ma Y, Wang R, Wang D, Yu P (2016) Inhibitory activity evaluation and mechanistic studies of tetracyclic oxindole derivatives as α-glucosidase inhibitors. Eur J Med Chem 123:365–378. doi:10.1016/j.ejmech.2016.07.044
Acknowledgements
This work was supported by the State Ethnic Affairs Commission Research Projects (14XBZ021), Research Foundation of the Gansu Higher Education Institutions of China (2014B-006), and the Fundamental Research Funds for the Central Universities (NO.31920130035).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare they have no competing interests.
Additional information
Y. J. Qi and H. N. Lu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Qi, Y.J., Lu, H.N., Zhao, Y.M. et al. Probing the influence of carboxyalkyl groups on the molecular flexibility and the charge density of apigenin derivatives. J Mol Model 23, 70 (2017). https://doi.org/10.1007/s00894-017-3221-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3221-3