Abstract
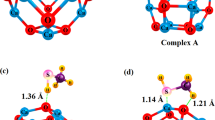
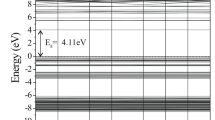
Metal doped ZnO nanomaterials have attracted considerable attention as a chemical sensor for toxic gases. Here, the electronic sensitivity of pristine and Sc-, Ti-, V-, Cr-, Mn-, and Fe-doped Zn12O12 nanoclusters toward CO gas is investigated using density functional theory calculations. It is found that replacing a Zn atom by a Sc or Ti atom does not change the sensitivity of cluster but doping V and Cr atoms significantly increase the sensitivity. Also, Mn, or Fe doping slightly improves the sensitivity. It is predicted that among all, the Cr-doped ZnO cluster may be the most favorable sensor for CO detection because its electrical conductivity considerably changes after the CO adsorption, thereby, generating an electrical signal. The calculated Gibbs free energy change for the adsorption of CO molecule on the Cr-doped cluster is about -51.2 kcal mol-1 at 298.15 K and 1 atm, and the HOMO-LUMO gap of the adsorbent is changed by about 117.8 %.





Similar content being viewed by others
References
Peyghan AA, Baei MT, Hashemian S, Torabi P (2013) Adsorption of CO molecule on AlN nanotubes by parallel electric field. J Mol Model 19:859–870
Omaye ST (2002) Metabolic modulation of carbon monoxide toxicity. Toxicology 180:139–150
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2011) Toxic CO detection by B12 N12 nanocluster. Microelectron J 42:1400–1403
Beheshtian J, Kamfiroozi M, Bagheri Z, Ahmadi A (2011) Computational study of CO and NO adsorption on magnesium oxide nanotubes. Phys E 44:546–549
Beheshtian J, Peyghan AA, Bagheri Z (2012) Selective function of Al12N12 nano-cage towards NO and CO molecules. Comput Mater Sci 62:71–74
Tikuisis P, Kane D, McLellan T, Buick F, Fairburn S (1992) Rate of formation of carboxyhemoglobin in exercising humans exposed to carbon monoxide. J Appl Physiol 72:1311–1319
Beheshtian J, Ahmadi Peyghan A, Bagheri Z (2013) Ab initio study of NH3 and H2 O adsorption on pristine and Na-doped MgO nanotubes. Struct Chem 24:165–170
Epifani M, Comini E, Díaz R, Genç A, Andreu T, Siciliano P, Morante JR (2016) Acetone sensors based on TiO2 nanocrystals modified with tungsten oxide species. J Alloys Compd 665:345–351
Beheshtian J, Baei MT, Bagheri Z, Peyghan AA (2012) Co-adsorption of CO molecules at the open ends of MgO nanotubes. Struct Chem 23:1981–1986
Degler D, Barz N, Dettinger U, Peisert H, Chassé T, Weimar U, Barsan N (2016) Extending the toolbox for gas sensor research: Operando UV/vis diffuse reflectance spectroscopy on SnO2-based gas sensors. Sensors Actuators B Chem 224:256–259
Peyghan AA, Yourdkhani S (2014) Capture of carbon dioxide by a nanosized tube of BeO: a DFT study. Struct Chem 25:419–426
Kim S, Park S, Park S, Lee C (2015) Acetone sensing of Au and Pd-decorated WO3 nanorod sensors. Sensors Actuators B Chem 209:180–185
Samadizadeh M, Rastegar SF, Peyghan AA (2015) The electronic response of nano-sized tube of BeO to CO molecule: a density functional study. Struct Chem 26:809–814
Beheshtian J, Peyghan AA, Bagheri Z (2013) Sensing behavior of Al-rich AlN nanotube toward hydrogen cyanide. J Mol Model 19(6):2197–2203
Beheshtian J, Baei MT, Peyghan AA, Bagheri Z (2012) Electronic sensor for sulfide dioxide based on AlN nanotubes: a computational study. J Mol Model 18:4745–4750
Goodarzi Z, Maghrebi M, Zavareh AF, Mokhtari-Hosseini Z-B, Ebrahimi-hoseinzadeh B, Zarmi AH, Barshan-tashnizi M (2015) Evaluation of nicotine sensor based on copper nanoparticles and carbon nanotubes. J Nanostruct Chem 5:237–242
Ahmadi Peyghan A, Hadipour NL, Bagheri Z (2013) Effects of Al doping and double-antisite defect on the adsorption of HCN on a BC2N nanotube: density functional theory studies. J Phys Chem C 117(5):2427–2432
Mahdavian L (2012) Simulation of SnO2/WO3 nanofilms for alcohol of gas sensor based on metal dioxides: MC and LD studies. J Nanostruct Chem 3:1–9
Soltani A, Ahmadi Peyghan A, Bagheri Z (2013) H2O2 adsorption on the BN and SiC nanotubes: a DFT study. Phys E 48:176–180
Noei M, Ebrahimikia M, Saghapour Y, Khodaverdi M, Salari AA, Ahmadaghaei N (2015) Removal of ethyl acetylene toxic gas from environmental systems using AlN nanotube. J Nanostruct Chem 5:213–217
Peyghan AA, Noei M, Yourdkhani S (2013) Al-doped graphene-like BN nanosheet as a sensor for para-nitrophenol: DFT study. Superlattice Microst 59:115–122
Saha M, Das S (2014) Fabrication of a nonenzymatic cholesterol biosensor using carbon nanotubes from coconut oil. J Nanostruct Chem 4:1–9
Beheshtian J, Peyghan AA, Noei M (2013) Sensing behavior of Al and Si doped BC3 graphenes to formaldehyde. Sensors Actuators B Chem 181:829–834
Beheshtian J, Peyghan AA, Bagheri Z (2012) Nitrate adsorption by carbon nanotubes in the vacuum and aqueous phase. Monatsh Chem 143:1623–1626
Beheshtian J, Peyghan AA, Bagheri Z (2012) Adsorption and dissociation of Cl2 molecule on ZnO nanocluster. Appl Surf Sci 258:8171–8176
Galstyan V, Comini E, Baratto C, Faglia G, Sberveglieri G (2015) Nanostructured ZnO chemical gas sensors. Ceram Int 41(10, Part B):14239–14244
Peyghan AA, Laeen SP, Aslanzadeh SA, Moradi M (2014) Hydrogen peroxide reduction in the oxygen vacancies of ZnO nanotubes. Thin Solid Films 556:566–570
Ameen S, Park D-R, Shaheer Akhtar M, Shin HS (2016) Lotus-leaf like ZnO nanostructures based electrode for the fabrication of ethyl acetate chemical sensor. Mater Lett 164:562–566
Peyghan AA, Noei M (2014) The alkali and alkaline earth metal doped ZnO nanotubes: DFT studies. Phys B Condens Matter 432:105–110
Rocha LSR, Foschini CR, Silva CC, Longo E, Simões AZ (2016) Novel ozone gas sensor based on ZnO nanostructures grown by the microwave-assisted hydrothermal route. Ceram Int 42(3):4539–4545
Peyghan AA, Rastegar SF, Bagheri Z (2015) Selective detection of F2 in the presence of CO, N2, O2, and H2 molecules using a ZnO nanocluster. Monatshefte für Chemie-Chem Mon 146(8):1233–1239
Salimi H, Peyghan AA, Noei M (2015) Adsorption of formic acid and formate anion on ZnO nanocage: a DFT study. J Clust Sci 26(2):609–621
Shirini F, Abedini M, Zamani S, Fallah Moafi H (2015) Introduction of W-doped ZnO nanocomposite as a new and efficient nanocatalyst for the synthesis of biscoumarins in water. J Nanostruct Chem 5:123–130
Benramache S, Arif A, Belahssen O, Guettaf A (2013) Study on the correlation between crystallite size and optical gap energy of doped ZnO thin film. J Nanostruct Chem 3:1–6
Hongsith N, Viriyaworasakul C, Mangkorntong P, Mangkorntong N, Choopun S (2008) Ethanol sensor based on ZnO and Au-doped ZnO nanowires. Ceram Int 34:823–826
Sahay P, Nath R (2008) Al-doped ZnO thin films as methanol sensors. Sensors Actuators B Chem 134(2):654–659
Zhu G, Xu H, Liu Y, Xu X, Ji Z, Shen X, Xu Z (2012) Enhanced gas sensing performance of Co-doped ZnO hierarchical microspheres to 1,2-dichloroethane. Sensors Actuators B Chem 166–167:36–43
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
O’boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package‐independent computational chemistry algorithms. J Comput Chem 29:839–845
Bacskay GB (1981) A quadratically convergent Hartree-Fock (QC-SCF) method. Application to closed shell systems. Chem Phys 61:385–404
Zare K, Shadmani N, Pournamdari E (2013) DFT/NBO study of Nanotube and Calixarene with anti-cancer drug. J Nanostruct Chem 3:1–6
Peyghan AA, Moradi M (2014) DFT study of ozone dissociation on BC3 graphene with Stone–Wales defects. J Mol Model 20:1–7
Zare K, Shadmani N (2013) Comparison of drug delivery systems: nanotube and p- sulphonatocalix[4]arene, by density functional theory. J Nanostruct Chem 3(1):1–6. doi:10.1186/2193-8865-3-72
Peköz R, Erkoç Ş (2008) Density functional theory study on the structural properties and energetics of microclusters. Phys E 40(9):2921–2930
Nagarajan V, Chandiramouli R, Sriram S, Gopinath P (2014) Quantum chemical studies on the structural and electronic properties of nickel sulphide and iron sulphide nanoclusters. J Nanostruct Chem 4(1):1–16
Chen L, Xu C, Zhang X-F, Zhou T (2009) Raman and infrared-active modes in MgO nanotubes. Phys E 41(5):852–855
Hesabi M, Hesabi M (2013) The interaction between carbon nanotube and skin anti-cancer drugs: a DFT and NBO approach. J Nanostruct Chem 3:1–6
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Birajdar SD, Khirade PP, Bhagwat V, Humbe AV, Jadhav K (2016) Synthesis, structural, morphological, optical and magnetic properties of Zn 1− x Co x O (0≤ x≤ 0.36) nanoparticles synthesized by sol gel-auto combustion method. Journal of Alloys and Compounds doi:10.1016/j.jallcom.2016.05.043
Seetawan U, Jugsujinda S, Seetawan T, Ratchasin A, Euvananont C, Junin C, Thanachayanont C, Chainaronk P (2011) Effect of calcinations temperature on crystallography and nanoparticles in ZnO disk. Mater Sci Appl 2:1302–1307
Beheshtian J, Baei MT, Peyghan AA (2012) Theoretical study of CO adsorption on the surface of BN, AlN, BP and AlP nanotubes. Surf Sci 606(11):981–985
Hadipour NL, Ahmadi Peyghan A, Soleymanabadi H (2015) Theoretical study on the Al-doped ZnO nanoclusters for CO chemical sensors. J Phys Chem C 119:6398–6404
Acknowledgments
This work was authorized by Technical Vocational University and supported by Iran Nanotechnology Initiative Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aslanzadeh, S. Transition metal doped ZnO nanoclusters for carbon monoxide detection: DFT studies. J Mol Model 22, 160 (2016). https://doi.org/10.1007/s00894-016-3032-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3032-y