Abstract
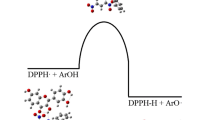
The stronger antioxidant capacity of the flavonoid quercetin (Q) compared with taxifolin (dihydroquercetin, T) has been the subject of previous experimental and theoretical studies. Theoretical work has focused on the analysis of hydrogen bond dissociation energies (BDE) of the OH phenolic groups, but consider mechanisms that only involve the transfer of one hydrogen atom. In the present work we consider other mechanisms involving a second hydrogen transfer in reactions with free radicals. The relative stability of the radicals formed after the first hydrogen transfer reaction is considered in discussing the antioxidant activity of Q and T. In terms of global and local theoretical reactivity descriptors, we propose that the radical arising from Q should be more persistent in the environment and with the capability to react with a second radical by hydrogen transfer, proton transfer and electron transfer mechanisms. These mechanisms could be responsible of the stronger antioxidant capacity of Q.




Similar content being viewed by others
References
Ross JA, Kasum CM (2002) Annu Rev Nutr 22:19–34
Hakkinen SH, Karenlampi SO, Heinonen IM, Mykkanen HM, Torronen AR (1999) J Agric Food Chem 47:2274–2279
Vinson JA, Dabbagh YA, Serry MM, Jang JH (1995) J Agric Food Chem 43:2800–2802
RiceEvans CA, Miller NJ, Paganga G (1996) Free Radical Biol Med 20:933–956
Burton GW, Doba T, Gabe EJ, Hughes L, Lee FL, Prasad L, Ingold KU (1985) J Am Chem Soc 107:7053–7065
de Heer MI, Mulder P, Korth HG, Ingold KU, Lusztyk J (2000) J Am Chem Soc 122:2355–2360
Jovanovic SV, Steenken S, Hara Y, Simic MG (1996) J Chem Soc Perkin 2:2497–2504
Jovanovic SV, Steenken S, Tosic M, Marjanovic B, Simic MG (1994) J Am Chem Soc 116:4846–4851
Foti MC, Daquino C, Geraci C (2004) J Org Chem 69:2309–2314
Litwinienko G, Ingold KU (2003) J Org Chem 68:3433–3438
Zhang HY, Ji HF (2006) New J Chem 30:503–504
Anouar E, Kosinova P, Kozlowski D, Mokrini R, Duroux JL, Trouillas P (2009) Phys Chem Chem Phys 11:7659–7668
Leopoldini M, Marino T, Russo N, Toscano M (2004) J Phys Chem A 108:4916–4922
Leopoldini M, Marino T, Russo N, Toscano M (2004) Theor Chem Acc 111:210–216
Leopoldini M, Pitarch IP, Russo N, Toscano M (2004) J Phys Chem A 108:92–96
Leopoldini M, Russo N, Chiodo S, Toscano M (2006) J Agric Food Chem 54:6343–6351
Wright JS, Johnson ER, DiLabio GA (2001) J Am Chem Soc 123:1173–1183
Leopoldini M, Russo N, Toscano M (2011) Food Chem 125:288–306
Russo N, Toscano M, Uccella N (2000) J Agric Food Chem 48:3232–3237
Priyadarsini I, Naik G, Mohan H, Maity D (2003) Free Radical Biol Med 35:8
Lemanska K, Szymusiak H, Tyrakowska B, Zielinski R, Soffers A, Rietjens I (2001) Free Radical Biol Med 31:869–881
Lucarini M, Peduli GF, Guerra M (2004) Chem-Eur J 10:933–939
DiLabio GA, Pratt DA, LoFaro AD, Wright JS (1999) J Phys Chem A 103:1653–1661
Trouillas P, Fagnere C, Lazzaroni R, Calliste C, Marfak A, Duroux JL (2004) Food Chem 88:571–582
Trouillas P, Marsal P, Siri D, Lazzaroni R, Duroux JL (2006) Food Chem 97:679–688
Zhang HY, Sung YM, Wang XL (2003) Chem-Eur J 9:502–508
Zhang HY (2004) New J Chem 28:1284–1285
Vanacker S, Degroot MJ, Vandenberg DJ, Tromp M, Donneopdenkelder G, VanderVijgh WJF, Bast A (1996) Chem Res Toxicol 9:1305–1312
Miller NJ, Riceevans C, Davies MJ, Gopinathan V, Milner A (1993) Clin Sci 84:407–412
Riceevans C, Miller NJ (1994) Methods Enzymol 234:279–293
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793–1873
Parr RG, Yang WT (1984) J Am Chem Soc 106:4049–4050
Parr RG, Von Szentpaly L, Liu SB (1999) J Am Chem Soc 121:1922–1924
Pearson RG (1963) J Am Chem Soc 85:3533–3543
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7516
Sen KD, Mingos DMP (1993) Chemical hardness: structure and Bonding. Springer, Berlin
Pearson RG (1997) Chemical Hardness. Wiley-VCH, New York
Parr RG, Yang W (1989) Density Functional Theory of atoms and Molecules. Oxford University Press, Oxford
Parkinson CJ, Mayer PM, Radom L (1999) J Chem Soc Perkin 2:2305–2313
Fuentealba P, Florez E, Tiznado W J Chem Theory Comput 6:1470–1478 -
Tiznado W, Chamorro E, Contreras R, Fuentealba P (2005) J Phys Chem A 109:3220–3224
Chamorro E, Duque M, Cardenas C, Santos C, Tiznado W, Fuentealba P (2005) J Chem Sci 117:419–424
Florez E, Tiznado W, Mondragon F, Fuentealba P (2005) J Phys Chem A 109:7815–7821
Tiznado W, Oña OB, Bazterra VE, Caputo MC, Facelli JC, Ferraro MB, Fuentealba P (2005) J Chem Phys 123
Tiznado W, Oña OB, Caputo MC, Ferraro MB, Fuentealba P (2009) J Chem Theory Comput 5:2265–2273
Chandanshive JZ, Bonini BF, Tiznado W, Escobar CA, Caballero J, Femoni C, Fochi M, Franchini MC Eur. J Org Chem:4806–4813
Osorio E, Ferraro MB, Ona OB, Cardenas C, Fuentealba P, Tiznado W J Chem Theory Comput 7:3995–4001
Osorio E, Ferraro MB, Ona OB, Cardenas C, Fuentealba P, Tiznado W J Chem Theory Comput 7:3995–4001
Chattaraj PK, Maiti B, Sarkar U (2003) J Phys Chem A 107:4973–4975
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1993) J Chem Phys 98:5648–5652
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–654
McLean AD, Chandler GS (1980) J Chem Phys 72:5639–5648
Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03. Gaussian, Inc, Wallingford, CT
Kohout M (2008) DGrid. Radebeul, Germany
Mulder P, Korth HG, Ingold KU (2005) Helv Chim Acta 88:370–374
Acknowledgments
This work was partially funded by the Millennium Scientific Initiative (Ministerio de Economía, Fomento y Turismo) (Grant P10-035-F), Fondo Nacional de Desarrollo Científico y Tecnológico (Grants: 11090431 and 11110241) and The Research Office of the University of Medellín, project 626. E.O. thanks to the Fondo de atracción a Postdoctorado, Universidad de Talca.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osorio, E., Pérez, E.G., Areche, C. et al. Why is quercetin a better antioxidant than taxifolin? Theoretical study of mechanisms involving activated forms. J Mol Model 19, 2165–2172 (2013). https://doi.org/10.1007/s00894-012-1732-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1732-5