Abstract
In our quest to explore molecules with chemically significant regions where the Fukui function is negative, we explored reactions where the frontier orbital that indicates the sites for electrophilic attack is not the highest occupied molecular orbital. The highest occupied molecular orbital (HOMO) controls the location of the regions where the Fukui function is negative, supporting the postulate that negative values of the Fukui function are associated with orbital relaxation effects and nodal surfaces of the frontier orbitals. Significant negative values for the condensed Fukui function, however, were not observed.

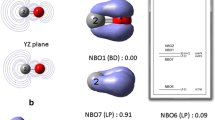
The −10−5isosurface of \( {f^{-}}\left( \mathbf{r} \right) \)(opaque silver surface) traces the nodal regions of the HOMO (translucent colored lobes, with different colors for different phases) of the phenoxide anion





Similar content being viewed by others
References
Ayers PW, Morrison RC, Roy RK (2002) Variational principles for describing chemical reactions: condensed reactivity indices. J Chem Phys 116:8731–8744
Melin J, Ayers PW, Ortiz JV (2007) Removing electrons can increase the electron density: a computational study of negative Fukui functions. J Phys Chem A 111:10017–10019
Bultinck P, Clarisse D, Ayers PW, Carbo-Dorca R (2011) The Fukui matrix: a simple approach to the analysis of the Fukui function and its positive character. Phys Chem Chem Phys 13:6110–6115
Ayers PW (2006) Can one oxidize an atom by reducing the molecule that contains it? Phys Chem Chem Phys 8:3387–3390
Bultinck P, Carbó-Dorca R (2003) Negative and infinite Fukui functions: the role of diagonal dominance in the hardness matrix. J Math Chem 34:67–74
Bultinck P, Carbó-Dorca R, Langenaeker W (2003) Negative Fukui functions: new insights based on electronegativity equalization. J Chem Phys 118:4349–4356
Bultinck P, Van Neck D, Acke G, Ayers PW (2012) Influence of electron correlation and degeneracy on the Fukui matrix and extension of frontier molecular orbital theory to correlated quantum chemical methods. Phys Chem Chem Phys 14:2408–2416
Bartolotti LJ, Ayers PW (2005) An example where orbital relaxation is an important contribution to the Fukui function. J Phys Chem A 109:1146–1151
Langenaeker W, Demel K, Geerlings P (1991) Quantum-chemical study of the Fukui function as a reactivity index. 2. Electrophilic substitution on mono-substituted benzenes. THEOCHEM J Mol Struct 80:329–342
Flurchick K, Bartolotti L (1995) Visualizing properties of atomic and molecular-systems. J Mol Graph 13(1):10–13
Petrini D, Larsson K (2008) Origin of the reactivity on the nonterminated (100), (110), and (111) diamond surfaces: an electronic structure DFT study. J Phys Chem C 112(37):14367–14376
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1873
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comput Chem 20:129–154
Liu SB (2009) Conceptual density functional theory and some recent developments. Acta Phys-Chim Sin 25:590–600
Gazquez JL (2008) Perspectives on the density functional theory of chemical reactivity. J Mex Chem Soc 52:3–10
Ayers PW, Anderson JSM, Bartolotti LJ (2005) Perturbative perspectives on the chemical reaction prediction problem. Int J Quantum Chem 101:520–534
Chattaraj PK, Sarkar U, Roy DR (2006) Electrophilicity index. Chem Rev 106:2065–2091
Johnson PA, Bartolotti LJ, Ayers PW, Fievez T, Geerlings P (2012) Charge density and chemical reactivity: a unified view from conceptual DFT. In: Gatti C, Macchi P (eds) Modern charge density analysis. Springer, New York, pp 715–764
Ayers PW, Yang WT, Bartolotti LJ (2009) Fukui function. In: Chattaraj PK (ed) Chemical reactivity theory: a density functional view. CRC, Boca Raton, pp 255–267
Ayers PW, Levy M (2000) Perspective on “Density functional approach to the frontier-electron theory of chemical reactivity” by Parr RG, Yang W (1984). Theor Chem Acc 103:353–360
Parr RG, Yang WT (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc 106:4049–4050
Yang WT, Parr RG, Pucci R (1984) Electron density, Kohn-Sham frontier orbitals, and Fukui functions. J Chem Phys 81:2862–2863
Cohen MH, Ganduglia-Pirovano MV (1994) Electronic and nuclear chemical reactivity. J Chem Phys 101:8988–8997
Ayers PW, Parr RG (2000) Variational principles for describing chemical reactions: the Fukui function and chemical hardness revisited. J Am Chem Soc 122:2010–2018
Chattaraj PK, Cedillo A, Parr RG (1995) Variational method for determining the Fukui function and chemical hardness of an electronic system. J Chem Phys 103:7645–7646
Perdew JP, Parr RG, Levy M, Balduz JL Jr (1982) Density-functional theory for fractional particle number: derivative discontinuities of the energy. Phys Rev Lett 49:1691–1694
Yang WT, Zhang YK, Ayers PW (2000) Degenerate ground states and fractional number of electrons in density and reduced density matrix functional theory. Phys Rev Lett 84:5172–5175
Ayers PW (2008) The continuity of the energy and other molecular properties with respect to the number of electrons. J Math Chem 43:285–303
Ayers PW, Yang W (2003) Density functional theory. In: Bultinck P, de Winter H, Langenaeker W, Tollenaere JP (eds) Computational medicinal chemistry for drug discovery. Dekker, New York, pp 571–616
Min KS, DiPasquale AG, Rheingold AL, White HS, Miller JS (2009) Observation of redox-induced electron transfer and spin crossover for dinuclear cobalt and iron complexes with the 2,5-Di-tert-butyl-3,6-dihydroxy-1,4-benzoquinonate bridging ligand. J Am Chem Soc 131:6229–6236
Miller JS, Min KS (2009) Oxidation leading to reduction: Redox-Induced Electron Transfer (RIET). Angew Chem Int Ed 48:262–272
Paul N, Samanta S, Goswami S (2010) Redox induced electron transfer in Doublet Azo-Anion Diradical Rhenium(II) Complexes. Characterization of complete electron transfer series. Inorg Chem 49:2649–2655
Kaim W (2011) Manifestations of noninnocent ligand behavior. Inorg Chem 50:9752–9765
Yang WT, Mortier WJ (1986) The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J Am Chem Soc 108:5708–5711
Bultinck P, Fias S, Alsenoy CV, Ayers PW, Carbó-Dorca R (2007) Critical thoughts on computing atom condensed Fukui functions. J Chem Phys 127:034102
Tiznado W, Chamorro E, Contreras R, Fuentealba P (2005) Comparison among four different ways to condense the Fukui function. J Phys Chem A 109:3220–3224
Bulat FA, Chamorro E, Fuentealba P, Toro-Labbé A (2004) Condensation of frontier molecular orbital Fukui functions. J Phys Chem A 108:342–349
Fuentealba P, Perez P, Contreras R (2000) On the condensed Fukui function. J Chem Phys 113:2544–2551
Contreras RR, Fuentealba P, Galvan M, Perez P (1999) A direct evaluation of regional Fukui functions in molecules. Chem Phys Lett 304:405–413
da Silva RR, Ramalho TC, Santos JM, Figueroa-Villar JD (2006) Reply to “comment on the paper ‘on the limits of highest-occupied molecular orbital driven reactions: the frontier effective-for-reaction molecular orbital concept”. J Phys Chem A 110(36):10653–10654
da Silva RR, Ramalho TC, Santos JM, Figueroa-Villar JD (2006) On the limits of highest-occupied molecular orbital driven reactions: the frontier effective-for-reaction molecular orbital concept. J Phys Chem A 110(3):1031–1040
Maksic ZB, Vianello R (2006) Comment on the paper “on the limits of highest-occupied molecular orbital driven reactions: the frontier effective-for-reaction molecular orbital concept”. J Phys Chem A 110(36):10651–10652
Anderson JSM, Melin J, Ayers PW (2007) Conceptual density-functional theory for general chemical reactions, including those that are neither charge nor frontier-orbital controlled. I. Theory and derivation of a general-purpose reactivity indicator. J Chem Theory Comput 3:358–374
Anderson JSM, Melin J, Ayers PW (2007) Conceptual density-functional theory for general chemical reactions, including those that are neither charge- nor frontier-orbital-controlled. 2. Application to molecules where frontier molecular orbital theory fails. J Chem Theory Comput 3:375–389
Politzer P, Murray JS, Bulat FA (2010) Average local ionization energy: a review. J Mol Model 16(11):1731–1742
Becke AD (1993) Density-functional thermochemistry. 3. The role of exact exchange. J Chem Phys 98:5648–5652
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys Rev A 38:3098–3100
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Miehlich B, Savin A, Stoll H, Preuss H (1989) Results obtained with the correlation-energy density functionals of Becke and Lee, Yang and Parr. Chem Phys Lett 157(3):200–206
Frisch MJ et al (2004) Gaussian03, Revision D.01. Gaussian, Wallingford
Reed AE, Weinstock RB, Weinhold F (1985) Natural-population analysis. J Chem Phys 83(2):735–746
Breneman CM, Wiberg KB (1990) Determining atom-centered monopoles from molecular electrostatic potentials - the need for high sampling density in formamide conformational-analysis. J Comput Chem 11(3):361–373
Wintner A (1948) On the counting of nodal curves and surfaces. J Chem Phys 16:405–406
Klopman G (1968) Chemical reactivity and the concept of charge and frontier-controlled reactions. J Am Chem Soc 90:223–234
Melin J, Aparicio F, Subramanian V, Galvan M, Chattaraj PK (2004) Is the Fukui function a right descriptor of hard-hard interactions? J Phys Chem A 108:2487–2491
Ayers PW, Parr RG, Pearson RG (2006) Elucidating the hard/soft acid/base principle: a perspective based on half-reactions. J Chem Phys 124:194107
Acknowledgments
P.W.A. thanks the Canada Research Chairs and Natural Sciences and Engineering Research Council of Canada (NSERC) for funding. Computational facilities were provided by Sharcnet. E.E. and A.T.L. acknowledge financial support from Fondecyt through project N°1090460. C.C. acknowledge financial support from Fondecyt through project No 11090013 and also by Financiamiento basal para centros científicos y tecnológicos de excelencia. J.S.M.A. would like to thank NSERC and The Japan Society for the Promotion of Science (JSPS) for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Echegaray, E., Cárdenas, C., Rabi, S. et al. In pursuit of negative Fukui functions: examples where the highest occupied molecular orbital fails to dominate the chemical reactivity. J Mol Model 19, 2779–2783 (2013). https://doi.org/10.1007/s00894-012-1637-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1637-3