Abstract
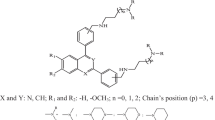
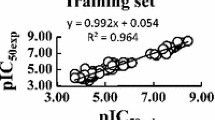
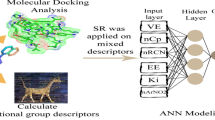
This work investigates neural network models for predicting the trypanocidal activity of 28 quinone compounds. Artificial neural networks (ANN), such as multilayer perceptrons (MLP) and Kohonen models, were employed with the aim of modeling the nonlinear relationship between quantum and molecular descriptors and trypanocidal activity. The calculated descriptors and the principal components were used as input to train neural network models to verify the behavior of the nets. The best model for both network models (MLP and Kohonen) was obtained with four descriptors as input. The descriptors were T5 (torsion angle), QTS1 (sum of absolute values of the atomic charges), VOLS2 (volume of the substituent at region B) and HOMO−1 (energy of the molecular orbital below HOMO). These descriptors provide information on the kind of interaction that occurs between the compounds and the biological receptor. Both neural network models used here can predict the trypanocidal activity of the quinone compounds with good agreement, with low errors in the testing set and a high correctness rate. Thanks to the nonlinear model obtained from the neural network models, we can conclude that electronic and structural properties are important factors in the interaction between quinone compounds that exhibit trypanocidal activity and their biological receptors. The final ANN models should be useful in the design of novel trypanocidal quinones having improved potency.

Compound component maps, where each map shows the calculated descriptors







Similar content being viewed by others
References
Siles R, Chen S, Zhou M, Pinney KG, Trawick ML (2006) Bioorg Med Chem Lett 16:4405–4409
Batista R, Humberto JL, Chiari E, Oliveira AB (2007) Bioorg Med Chem Lett 15:381–391
Bauer H, Massey V, Arscott LD, Schirmer RH, Ballou DP, Williams CH (2003) J Biol Chem 278:33020–33028
Li ZL, Fennie MW, Ganem B, Hancock MT, Kobaslija M, Rattendi D, Bacchi CJ, O’Sullivan M (2001) Bioorg Med Chem Lett 11:251–254
del Corral JMM, Castro MA, Oliveira AB, Gualberto SA, Cuevas C, San Feliciano A (2006) Bioorg Med Chem 14:7231–7240
Winkler DA, Burden FR (2004) Drug Discov Today 2:104–111
Katritzky AR, Pacureanu LM, Slavov S, Dobchev DA, Karelson M (2006) Bioorg Med Chem 14:6933–6939
Fernández M, Caballero J (2006) Bioorg Med Chem 14:280–294
Selzer P, Ertl PJ (2006) Chem Inf Model 46:2319–2323
Goulart MOF, Zani CL, Tonholo J, Freitas LR, de Abreu FC, Oliveira AB, Raslan DS, Starling S, Chiari E (1997) Bioorg Med Chem Lett 7:2043–2048
Becke AD (1993) J Chem Phys 98:5648–5652
El-Azhary AA, Sutter HU (1996) J Phys Chem 100:15056–15063
Turecek F (1998) J Phys Chem A 102:4703–4713
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreyen Jr T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Octhterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al- Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision C.02. Gaussian, Pittsburgh PA
Breneman CM, Wiberg KB (1990) J Comput Chem 11:361–373
Wang R, Fu Y, Lai L (1997) J Inf Comput Sci 37:615–621
Todeschini R, Gramatica P (1998) Perspect Drug Discov Des 9:355–380
Skrobot VL, Castro EVR, Pereira RCC, Pasa VMD, Fortes ICP (2007) Energy Fuels 21:3394–3400
González-Arjona D, López-Pérez A, González AG (2002) Talanta 56:79–90
Molfetta FA, Bruni AT, Honório KM, da Silva ABF (2005) Eur J Med Chem 40:329–338
Bykov VA, Popov PI, Pleteneva TV, Anisimova IE, Syroeshkin AV (2004) Pharm Chem J 38:243–249
Kubinyi H, Folkers G, Martin YC (2002) 3D QSAR in drug design, vol 2. Kluwer, New York
Marini F, Zupan J, Magri AL (2005) Anal Chim Acta 544:306–314
Kawakami J, Hoshi K, Ishiyama A, Miyagishima S, Sato K (2004) Chem Pharm Bull 52:751–755
Santo LLD, Galvão DS (1999) J Mol Struct (THEOCHEM) 464:273–279
Barone PMVB, Camilo A, Galvão DS (1996) Phys Rev Lett 77:1186–1189
Braga RS, Barone PMVB, Galvão DS (1999) J Mol Struct (THEOCHEM) 464:257–266
Rothenberg G, Sasson Y (1999) Tetrahedron 55:561–568
Acknowledgments
The authors would like to acknowledge CAPES, CNPq and FAPESP (Brazilian Agencies) for the financial support given to this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Molfetta, F.A., Angelotti, W.F.D., Romero, R.A.F. et al. A neural networks study of quinone compounds with trypanocidal activity. J Mol Model 14, 975–985 (2008). https://doi.org/10.1007/s00894-008-0332-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-008-0332-x