Abstract
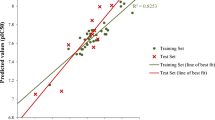
Artemisinin and some derivatives with activity against D-6 strains of Plasmodium falciparum were studied. Molecular electrostatic potential (MEP) maps were used in an attempt to identify key features of the compounds that are necessary for their activities, and then use those to propose new artemisinin derivatives. The partial least squares (PLS) method was then used to generate a predictive model. The PLS model with three latent variables explaining 88.9% of total variance, with Q2 = 0.839 and R2 = 0.935, was obtained for 15/6 compounds in the training/external validation set. For construction of the model, the most important descriptors were the highest occupied molecular orbital (HOMO) energy, atomic charges on the atoms O1 (Q1) and C3 (Q3), molecular volume (VOL), and hydrophilic index (HYF). From a set of 20 proposed artemisinin derivatives, one new compound (39) with higher antimalarial activity than the molecules initially studied was predicted. Synthesis of these new derivatives may follow the results of the MEP maps studied and the PLS modeling.

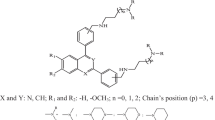
Molecular electrostatic potential (MEP) (values in au) map computed from electronic density to the novel proposed artemisinin derivative (39). The red color indicates the 1,2,4-trioxane ring in the region of most negative MEPdue to the peroxide linkage characteristic of compounds active against D-6 strains of Plasmodium falciparum.








Similar content being viewed by others
References
Bowman S, Lawson D, Basham D, Brow D, Chillingworth T, Churcher CM, Craig A, Davies RM, Devlin K, Feltwell T, Gentles S, Gwillinam R, Hamlin N, Harris D, Holroyd S, Hornsby T, Horrocks P, Jagels K, Jassal B, Kyes SJ, McLean S, Moule S, Mungall K, Murphy L, Olive RK, Quail MA, Rajadream M-A, Rutter S, Skelton J, Squares R, Squares S, Sulston JE, Whitehead S, Woodward JR, Newbold C, Barrell BG (1999) Nature 400:532–538
Pandey AV, Tekwani BL, Singh RL, Chaudan SV (1999) J Biol Chem 274:19383–19388
Kamchonwonggpaisan S, Samoff E, Meschinck SR (1997) Mol Biochem Parasitol 86:179–186
Olliaro P (2001) Pharmacol Ther 89:207–219
Ridley RG (2002) Nature 415:686–692
White NJ (2004) J Clin Invest 113:1084–1092
Arav-Boger R, Shapiro TA (2005) Annu Rev Pharmacol Toxicol 45:565–585
Cheng F, Shen J, Luo X, Zhu W, Gu J, Ji R, Jiang H, Chen K (2002) Bioorg Med Chem 10:2883–2891
Bhattacharjee AK, Hartell MG, Nichols DA, Hicks RP, Stanton B, van Hamont JE, Milhous WK (2004) Eur J Med Chem 39:59–67
Jefford CW (2001) Curr Med Chem 8:1803–1826
Haynes RK, Vonwiller SC (1997) Acc Chem Res 30:73–79
Bernardinelli G, Jefford CW, Maric D, Thomson C, Weber J (1994) Int J Quant Chem: Quant Biol Symp 21:117–131
Posner GH, Cumming JN, Ploypradith P, Oh CH (1995) J Am Chem Soc 117:5885–5886
Posner GH, Wang D, Cumming JN, Oh CH, French AN, Bodley AL, Shapiro TA (1995) J Med Chem 38:2273–2275
Haynes RK, Vonwiller SC (1996) Tetrahedron Lett 37:253–256
Rafiee MA, Hadipour NL, Naderi-manesh H (2005) J Chem Inf Model 45:366–370
Wu WM, Wu YK, Wu YL, Yao ZJ, Zhou CM, Li Y, Shan F (1998) J Am Chem Soc 120:3316–3325
Meshnick SR (2002) Int J Parasitol 32:1655–1660
Hynes RK, Krishna S (2004) Microb Infect 6:1339–1346
Kannan R, Kumar K, Sahal D, Kukreti S, Chauhan VS (2005) Biochem J 385:409–418
Kamchonwongpaisan S, Samoff E, Meschinck SR (1997) Mol Biochem Parasitol 86:179–186
Hon YL, Yang YZ, Meschnick SR (1998) Mol Biochem Parasitol 63:121–128
Wang DY, Wu YL (2000) Chem Commun 22:2193–2194
Hynes RK, Ho W-Y, Chen H-W, Fugmann B, Stetter J, Croft SL, Vivas L, Peters W, Robinson BR (2004) Angew Chem Int Ed 43:1381–1385
Hynes RK (2005) Angew Chem Int Ed 44:2064–2065
Pinheiro JC, Kiralj R, Ferreira MMC, Romero OAS (2003) QSAR Comb Sci 22:830–842
Roothaan CCJ (1951) Rev Mod Phys 23:69–89
Binkley JS, Pople JA, Hehre WJ (1980) J Am Chem Soc 102:939–946
Lin AJ, Zikry AB, Kyle DE (1997) J Med Chem 40:1396–1400
Lin AJ, Miller RE (1995) J Med Chem 38:764–770
Beebe KR, Pell JR, Seasholtt MB (1998) Chemometrics: a pratical guide. Wiley, New York, pp 81–278
Ferreira MMC (2002) J Braz Chem Soc 13:742–753
Kubinyi H (1993) QSAR: Hansch analysis and related approaches. In: Mannhold R, Krogsgaard-Larsen P, Timmerman H (eds) Methods and principles in medicinal chemistry, vol 1. VHC, Weinheim, pp 1–240
GaussView 1.0 (1997) Gaussian Inc., Pittsburgh, PA
Pinheiro JC, Ferreira MMC, Romero OAS (2001) J Mol Struct (Theochem) 572:35–44
Leban I, Golic L, Japelj M (1998) Acta Pharm Jugosl 38:71–77
Lisgarten JN, Potter BS, Bantuzeko C, Palmer RA (1998) J Chem Cryst 28:539–543
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Millam MA, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi Barone JV, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Salvador P, Dannenberg JJ, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Sefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, A-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andes JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2001) Gaussian 98 - Revision A.11. Gaussian Inc, Pittsburgh PA
Flukiger P, Luthi HP, Portmann S, Weber J (2000–2001) MOLEKEL. Swiss Center for Scientific Computing, Mano, Switzerland
Todeschine R, Gramatica P (1997) WHIM-3D 3.3. Milan
ChemPlus (2000) Modular extensions to hyperChem release 6.02, molecular modeling for windows. HyperCube Inc, Gainesvillem FL
Infometrix, Pirouette 3.01, Woodinville WA, 2001
Acknowledgments
We gratefully acknowledge the financial support of the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. The authors would like to thank the Instituto de Química-Araraquara for the use of the GaussView software, and the Swiss Center for Scientific Computing for the use of the MOLEKEL software. We employed computing facilities at the Centro Nacional de Processamento de Alto Desempenho-Universidade Estadual de Campinas and at the Laboratório de Química Teórica e Computacional-Universidade Federal do Pará.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cardoso, F.J.B., de Figueiredo, A.F., da Silva Lobato, M. et al. A study on antimalarial artemisinin derivatives using MEP maps and multivariate QSAR. J Mol Model 14, 39–48 (2008). https://doi.org/10.1007/s00894-007-0249-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-007-0249-9