Abstract
A three dimensional structural model of Glutathione-S-transferase (GST) of the lymphatic filarial parasite Wuchereria bancrofti (wb) was constructed by homology modeling. The three dimensional X-ray crystal structure of porcine π-class GST with PDB ID: 2gsr-A chain protein with 42% sequential and functional homology was used as the template. The model of wbGST built by MODELLER6v2 was analyzed by the PROCHECK programs. Ramachandran plot analysis showed that 93.5% of the residues are in the core region followed by 5.4 and 1.1% residues in the allowed and generously allowed regions, respectively. None of the non-glycine residues is in disallowed regions. The PROSA II z-score and the energy graph for the final model further confirmed the quality of the modeled structure. The computationally modeled three-dimensional (3D) structure of wbGST has been submitted to the Protein Data Bank (PDB) (PDB ID: 1SFM and RCSB ID: RCSB021668). 1SFM was used for docking with GST inhibitors by Hex4.2 macromolecular docking using spherical polar Fourier correlations.
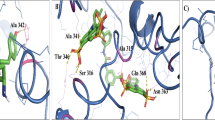
Figure: A three-dimensional (3D) structure of Glutathione-S-transferase (GST) of the lymphatic filarial parasite Wuchereria bancrofti (wb) was constructed by homology modeling. This modeled 3D structure of wbGST has been submitted to the Protein Data Bank (PDB) (PDB ID: 1SFM and RCSB ID: RCSB021668).






Similar content being viewed by others
References
WHO/CDS/CPE/CEE/2002.28. Global programme to eliminate lymphatic filariasis
Michele AM, DeWight RW, John AT (1995) J Mol Biol 246:21–27
Harwaldt P, Rahlfs S, Becker K (2002) Biol Chem 383:821–830
Brophy PM, Campbell AM, van Eldik AJ, Teesdale-Spittle PH, Liebau E, Wang MF (2000) Bioorg Med Chem Lett 10:979–981
Rao UR, Salinas G, Mehta K, Klei TR (2000) Parasitol Res 86:908–915
Mathew N, Paily KP, Vanamail P, Kalyanasundaram AM, Balaraman K (2002) Drug Dev Res 56:33–39
Brophy PM, Pritchard DI (1994) Exp Parasitol 79:89–96
Precious WY, Barrett J (1989) J Parasitol Today 5:156–160
Liebau E, Wildenburg G, Brophy PM, Walter RD, Henkle-Duhrsen K (1996) Mol Biochem Parasitol 80:27–39
Wildenburg G, Liebau E, Henkle-Duhrsen K (1998) Exp Parasitol 88:34–42
Rathaur S, Fischer P, Domagalsky M, Walter RD, Liebau E (2003) Exp Parasitol 103:177–181
Campbell AM, van Eldik AJ, Liebau E, Barrett J, Brophy PM, Teesdale-Spittle PH, Wang MF (2001) Chem-Biol Interact 133:240–243
Johnson MS, Srinivasan N, Sowdhamini R, Blundell TL (1994) Crit Rev Biochem Mol Biol 29:1–68
Sali A (1995) Curr Opin Biotechnol 6:437–451
Rost B, Sander C (1996) Annu Rev Biophys Biomol Struct 25:113–136
Chothia C, Lest AM (1986) EMBO J 5:823–826
Sanchez R, Sali A (1997) Curr Opin Struct Biol 7:206–214
Altschul SF, Thomas LM, Alejandro A, Schäffer JZ, Zheng Z, Webb M, David JL (1997) Nucleic Acids Res 25:3389–3402
Dirr H, Reinemer P, Huber R (1994) J Mol Biol 243:72–92
Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, Gibson T (1994) J Nucleic Acids Res 22:4673–4680
Sali A, Blundell TL (1993) J Mol Biol 234:779–815
Sali A, Potterton L, Yuan F, van Vlijmen H, Karplus M (1995) Proteins 23:318–326
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) J Appl Crystallogr 26:283–291
Hooft RWW, Vriend G, Sander C, Abola EE (1996) Nature 381:272
Ramachandran GN, Ramakrishnan C, Sasisekharan V (1963) J Mol Biol 7:95–99
Morris AL, MacArthur MW, Hutchinson EG, Thornton JM (1992) Proteins 12:345–364
Sippl MJ (1993) Proteins 17:355–362
Guex N, Peitsch MC (1997) Electrophoresis 18:2714–2723
Ritchie DW, Kemp GJL (2000) Proteins: Struct Funct Genet 39:178–194
Gilliland GL (1993) Curr Opin Struct Biol 3:875–884
Acknowledgments
The authors are grateful to Dr. P.K. Das, Director, Vector Control Research Centre for providing the facility and his encouragement during the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nathan, S.T., Mathew, N., Kalyanasundaram, M. et al. Structure of glutathione S-transferase of the filarial parasite Wuchereria bancrofti: a target for drug development against adult worm. J Mol Model 11, 194–199 (2005). https://doi.org/10.1007/s00894-005-0234-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-005-0234-0