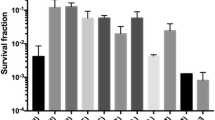
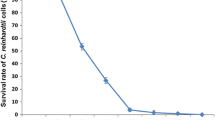
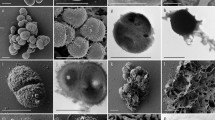
Abstract
Desiccation-tolerant cells must either protect their cellular components from desiccation-induced damage and/or repair it upon rewetting. Subcellular damage to the anhydrobiotic cyanobacterium Chroococcidiopsis sp. CCMEE 029 stored in the desiccated state for 4 years was evaluated at the single-cell level using fluorescent DNA strand breakage labelling, membrane integrity and potential related molecular probes, oxidant-sensing fluorochrome and redox dye. Covalent modifications of dried genomes were assessed by testing their suitability as PCR template. Results suggest that desiccation survivors avoid/and or limit genome fragmentation and genome covalent modifications, preserve intact plasma membranes and phycobiliprotein autofluorescence, exhibit spatially-reduced ROS accumulation and dehydrogenase activity upon rewetting. Damaged cells undergo genome fragmentation, loss of plasma membrane potential and integrity, phycobiliprotein bleaching, whole-cell ROS accumulation and lack respiratory activity upon rewetting. The co-occurrence of live and dead cells within dried aggregates of Chroococcidiopsis confirms that desiccation resistance is not a simple process and that subtle modifications to the cellular milieu are required to dry without dying. It rises also intriguing questions about the triggers of dead cells in response to drying. The capability of desiccation survivors to avoid and/or reduce subcellular damage, shows that protection mechanisms are relevant in the desiccation tolerance of this cyanobacterium.


Similar content being viewed by others
Abbreviations
- CM-H2DCFDA:
-
5-(and-6)-Chloromethyl-2′, dichlorodihydrofluorescein diacetate, acetyl ester
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DCF:
-
2′,7′-Dichlorofluorescein
- DiBAC4(3):
-
Bis-(1,3-dibutylbarbituric acid) trimethine oxonol
- FITC:
-
Fluorescein isothiocyanate
- HIP1:
-
Highly iterated palindromic sequences, type1
- INT:
-
2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium chloride
- PCD:
-
Programmed cell death
- STRR:
-
Short tandemly repeated repetitive
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling
References
Alpert P (2006) Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? J Exp Biol 209:1575–1584
Bassler BL, Losick R (2006) Bacterially speaking. Cell 125:237–246
Berman-Frank I, Bidle KD, Haramaty L, Falkowski PG (2004) The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnol Oceanogr 49:997–1005
Bidle KD, Falkowski PG (2004) Cell death in planktonic, photosynthetic microorganisms. Nat Rev Microbiol 2:643–655
Billi D, Potts M (2000) Life without water: responses of prokaryotes to desiccation. In: Storey KB, Storey JM (eds) Cell and molecular response to stress: environmental stressors and gene responses. Elsevier, Amsterdam, pp 181–192
Billi D, Potts M (2002) Life and death of dried prokaryotes. Res Microbiol 153:7–12
Billi D, Friedmann EI, Hofer KG, Grilli Caiola M, Ocampo-Friedmann R (2000) Ionizing-radiation resistance in the desiccation-tolerant cyanobacterium Chroococcidiopsis. Appl Environ Microbiol 66:1489–1492
Bruno L, Billi D, Albertano P (2005) Optimization of molecular techniques applied to the taxonomy of epilithic Leptolyngbya strains. Arch Hydrobiol Suppl Algol Stud 117:197–207
Cockell CS, Schuerger AC, Billi D, Friedmann EI, Panitz C (2005) Effects of a Simulated Martian UV Flux on the cyanobacterium, Chroococcidiopsis sp. 029. Astrobiology 5:127–140
Crowe JH (2007) Trehalose as a “chemical chaperone”: fact and fantasy. Adv Exp Med Biol 94:143–158
Daly MJ (2006) Modulating radiation resistance: insights based on defenses against reactive oxygen species in the radioresistant bacterium Deinococcus radiodurans. Clin Lab Med 26:491–504
Du J, Gebicki JM (2004) Proteins are major initial cell targets of hydroxyl free radicals. Int J Biochem Cell Biol 36:2334–2343
França MB, Panek AD, Eleutherio EC (2007) Oxidative stress and its effects during dehydration. Comp Biochem Physiol A Mol Integr Physiol 146:621–631
Fredrickson JK, Li SMW, Gaidamakova EK, Matrosova VY, Zhai M, Sulloway HM, Scholten JC, Brown MG, Balkwill DL, Daly MJ (2008) Protein oxidation: key to bacterial desiccation resistance? Int J Syst Evol Microbiol 2:393–403
Friedmann EI, Kappen L, Meyer MA, Nienow JA (1993) Long-term productivity in the cryptoendolithic communities of the Ross desert, Antarctica. Microb Ecol 25:51–69
Grilli Caiola M, Billi D (2007) Chroococcidiopsis from desert to Mars. In: Seckbach J (ed) Algae and cyanobacteria in extreme environments. Cellular origin, life in extreme habitats and astrobiology. Springer, Berlin, pp 553–568
Grilli Caiola M, Ocampo-Friedmann R, Friedmann EI (1993) Cytology of long-term desiccation in the cyanobacterium Chroococcidiopsis (Chroococcales). Phycologia 32:315–322
Grilli Caiola M, Billi D, Friedmann EI (1996a) Effect of desiccation on envelopes of the cyanobacterium Chroococcidiopsis sp. (Chroococcales). Eur J Phycol 31:97–105
Grilli Caiola M, Canini A, Ocampo-Friedmann R (1996b) Iron superoxide dismutase (Fe-SOD) localization in Chroococcidiopsis (Chroococcales, Cyanobacteria). Phycologia 35:90–94
He YY, Häder DP (2002) Reactive oxygen species and UV-B: effect on cyanobacteria. Photochem Photobiol Sci 1:729–736
Higo A, Katoh H, Ohmori K, Ikeuchi M, Ohmori M (2006) The role of a gene cluster for trehalose metabolism in dehydration tolerance of the filamentous cyanobacterium Anabaena sp. PCC 7120. J Microbiol 152:979–987
Katoh H, Asthana RK, Ohmori M (2004) Gene expression in the cyanobacterium Anabaena sp. PCC7120 under desiccation. Microb Ecol 47:164–174
Kolodkin-Gal I, Hazan R, Gaathon A, Carmeli S, Engelberg-Kulka H (2007) A linear pentapeptide is a quorum-sensing factor required for mazEF-mediated cell death in Escherichia coli. Science 318:652–655
Kranner I, Birtić S (2005) A modulating role for antioxidants in desiccation tolerance. Integr Comp Biol 45:734–740
Mattimore V, Battista JR (1996) Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol 178:633–637
Ning SB, Guo HL, Wang L, Song YC (2002) Salt stress induces programmed cell death in prokaryotic organism Anabaena. J Appl Microbiol 93:15–28
Potts M (1985) Protein synthesis and proteolysis in immobilized cells of cyanobacterium Nostoc commune UTEX 584 exposed to matric water stress. J Bacteriol 164:1025–1031
Potts M (1999) Mechanisms of desiccation tolerance in cyanobacteria. Eur J Phycol 34:319–328
Potts M (2001) Desiccation tolerance: a simple process? Trends Microbiol 9:553–559
Potts M, Slaughter SM, Hunneke FU, Garst JF, Helm RF (2005) Desiccation tolerance of prokaryotes: application of principles to human cells. Integr Comp Biol 45:800–809
Robinson NJ, Rutherford JC, Pocock MR, Cavet JS (2000) Metal metabolism and toxicity: repetitive DNA. In: Whitton BA, Potts M (eds) The ecology of cyanobacteria: their diversity in time and space. Kluwer, Dordrecht, pp 443–463
Shirkey B, Kovarcik DP, Wtight DJ, Wilmoth G, Prickett TF, Helm RF, Gregory EM, Potts M (2000) Active Fe-containing superoxide dismutase and abundant sodF mRNA in Nostoc commune (Cyanobacteria) after years of desiccation. J Bacteriol 182:189–197
Shirkey B, McMaster NJ, Smith SC, Wright DJ, Rodriguez H, Jaruga P, Birincioglu M, Helm RF, Potts M (2003) Genomic DNA of Nostoc commune (Cyanobacteria) becomes covalently modified during long-term (decades) desiccation but is protected from oxidative damage and degradation. Nucleic Acids Res 31:2995–3005
Tamaru Y, Takani Y, Yoshida T, Sakamoto T (2005) Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl Environ Microbiol 71:7327–7333
Tripathi SN, Shivali K, Shrivastava A (2007) Extraction and purification of an unusual phycoerythrin in a terrestrial desiccation tolerant cyanobacterium Lyngbya arboricola. J Appl Phycol 19:441–447
Warren-Rhodes KA, Rhodes KL, Pointing SB, Ewing SA, Lacap DC, Gómez-Silva B, Amundson R, Friedmann EI, McKay CP (2006) Hypolithic cyanobacteria, dry limit of photosynthesis, and microbial ecology in the hyperarid Atacama Desert. Microb Ecol 52:389–398
Wierzchos J, Ascaso C, McKay CP (2006) Endolithic cyanobacteria in halite rocks from the hyperarid core of the Atacama Desert. Astrobiology 6:415–422
Zimmermann R, Iturriaga R, Becker-Birck J (1978) Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol 36:926–935
Acknowledgments
I thank anonymous reviewers for helpful comments. This work was funded by the Italian Space Agency (MoMa project) and the Italian Ministry of Foreign Affairs (Direzione Generale per la Promozione e Cooperazione Culturale). Thanks are due to Dr. Palma Mattioli for image analysis and Roberto Targa for skilful assistance in image editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Driessen.
This paper is dedicated to the memory of E. Imre Friedmann and his wife Roseli, who pioneered researches on Chroococcidiopsis and life in desert environments.
Rights and permissions
About this article
Cite this article
Billi, D. Subcellular integrities in Chroococcidiopsis sp. CCMEE 029 survivors after prolonged desiccation revealed by molecular probes and genome stability assays. Extremophiles 13, 49–57 (2009). https://doi.org/10.1007/s00792-008-0196-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-008-0196-0