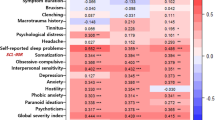
Abstract
Investigating the collective impact of psychometric properties and sleep quality on pain sensitivity in temporomandibular disorder (TMD) patients could improve clinical management strategies.
Objective
Assessing whether combined psychometric properties and sleep quality impact painful mechanical sensitivity and pain modulation in TMD patients.
Materials and methods
A cross-sectional study using secondary data analysis of 77 TMD patients and 101 controls. All participants completed questionnaires characterizing their psychometric profile (anxiety, depression, stress and catastrophizing) and sleep quality, alongside psychophysical tests for painful mechanical sensory (mechanical pain threshold (MPT), pressure pain threshold (PPT), and wind-up ratio (WUR)) and conditioned pain modulation (CPM). Participants were grouped into "High distress" or "Low distress" categories based on psychometric properties and sleep quality using hierarchical cluster and k-means analyses. Multiple linear regression evaluated the influence of TMD, age, and the distress cluster on MPT, WUR, PPT, and CPM in masseter and thenar muscles. Differences were statistically significant when p < 0.05.
Results
The presence of TMD was the strongest predictor of mechanical painful sensitivity in the trigeminal region (MPT[F(3,174) = 51.902;p < .001;R2 = .463]; TMD presence (β = -.682) / PPT[F(3,174) = 15.573;p < .001;R2 = .198] TMD presence (β = -.452), and extra-trigeminal (MPT[F(3,174) = 35.897;p < .001;R2 = .382] TMD (β = -.647) / CPM [F(3,174) = 4.106;p < .05;R2 = .050] TMD presence (β = .197). Furthermore, neither the high distress group nor the low distress group were able to significantly influence the variation of the values of any of the psychophysical variables evaluated (p > .05).
Conclusions
There is not a significant influence of impairment clusters based on psychological variables and sleep quality on painful mechanical sensitivity and pain modulation, regardless of the presence of TMD.
Clinical relevance
This outcome suggests that psychosocial factors and sleep quality may not play a decisive role in the sensory-discriminative aspect of pain, particularly concerning painful TMD.

Similar content being viewed by others
Data availability
The data that support the findings of this study will be available from the authors. The data used in the research project, whether raw, modified, groupings carried out by k-means clustering, are available through the open link:
https://docs.google.com/spreadsheets/d/12yyukoXzE0GkOKpYUEmSwj4QsX0x3vRC7KOi604zMSE/edit#gid=0
The data does not identify the volunteers, preserving their identity.
References
Romero-Reyes M, Klasser G, Akerman S (2023) An Update on Temporomandibular Disorders (TMDs) and Headache. Curr Neurol Neurosci Rep 23:561–570
Okeson JP, de Leeuw R (2011) Differential Diagnosis of Temporomandibular Disorders and Other Orofacial Pain Disorders. Dent Clin North Am 55:105–120
Crofford LJ (2015) Psychological aspects of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol 29:147–155
Benoliel R, Zini A, Zakuto A, Slutzky H, Haviv Y, Sharav Y, Almoznino G (2017) Subjective Sleep Quality in Temporomandibular Disorder Patients and Association with Disease Characteristics and Oral Health-Related Quality of Life. J Oral Facial Pain Headache 31:313–322. https://doi.org/10.11607/ofph.1824
Rener-Sitar K, John MT, Pusalavidyasagar SS, Bandyopadhyay D, Schiffman EL (2016) Sleep quality in temporomandibular disorder cases. Sleep Med 25:105–112. https://doi.org/10.1016/j.sleep.2016.06.031
Sullivan MJL, Bishop SR, Pivik J (1995) The Pain Catastrophizing Scale: Development and Validation
Nielsen MG, Ørnbøl E, Vestergaard M, Bech P, Larsen FB, Lasgaard M, Christensen KS (2016) The construct validity of the Perceived Stress Scale. J Psychosom Res 84:22–30. https://doi.org/10.1016/j.jpsychores.2016.03.009
Castro MM, Quarantini L, Batista-Neves S, Kraychete DC, Daltro C, Miranda-Scippa A (2006) Validade da escala hospitalar de ansiedade e depressão em pacientes com dor crônica [Validity of the hospital anxiety and depression scale in patients with chronic pain.]. Revista brasileira de anestesiologia 56(5):470–477. https://doi.org/10.1590/s0034-70942006000500005
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta psychiatrica Scandinavica 67(6):361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
Cheng ST, Chen PP, Chow YF, Chung JWY, Law ACB, Lee JSW, Leung EMF, Tam CWC (2019) The Pain Catastrophizing Scale-Short form: Psychometric properties and threshold for identifying high-risk individuals. Int Psychogeriatr 31:1665–1674. https://doi.org/10.1017/S1041610219000024
Siqueira Reis R, Ferreira Hino AA, RomÉlio Rodriguez AÑez C (2010) Perceived stress scale: Reliability and validity study in Brazil. J Health Psychol 15:107–114. https://doi.org/10.1177/1359105309346343
Sanders AE, Akinkugbe AA, Bair E, Fillingim RB, Greenspan JD, Ohrbach R, Dubner R, Maixner W, Slade GD (2016) Subjective Sleep Quality Deteriorates before Development of Painful Temporomandibular Disorder. Journal of Pain 17:669–677. https://doi.org/10.1016/j.jpain.2016.02.004
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research 28(2):193–213. https://doi.org/10.1016/0165-1781(89)90047-4
Bertolazi AN, Fagondes SC, Hoff LS, Dartora EG, da Silva Miozzo IC, de Barba MEF, Menna Barreto SS (2011) Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med 12:70–75. https://doi.org/10.1016/j.sleep.2010.04.020
Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W (2011) Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain 12. https://doi.org/10.1016/j.jpain.2011.08.007
Weaver KR, Griffioen MA, Klinedinst NJ, Galik E, Duarte AC, Colloca L, Resnick B, Dorsey SG, Renn CL (2022) Quantitative sensory testing across chronic pain conditions and use in special populations. Front Pain Res 2. https://doi.org/10.3389/fpain.2021.779068
Soares FFC, Ferreira DMAO, Raimundini AA, Dionísio TJ, dos Santos CF, Conti PCR, Costa YM, Bonjardim LR (2023) Influence of genetic polymorphisms on mechanical pain sensitivity and endogenous pain modulation of trigeminal and spinal areas. J Oral Rehabil 50:39–53. https://doi.org/10.1111/joor.13384
Kothari SF, Baad-Hansen L, Oono Y, Svensson P (2015) Somatosensory assessment and conditioned pain modulation in temporomandibular disorders pain patients. Pain 156:2545–2555. https://doi.org/10.1097/j.pain.0000000000000325
Cruz-Almeida Y, Fillingim RB (2014) Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med (United States) 15:61–72
Costa YM, de Araújo-Júnior ENS, Fiedler LS, de Souza PRJ, Silva LLCP, Ferreira DMAO, Conti PCR, Bonjardim LR (2019) Reproducibility of quantitative sensory testing applied to musculoskeletal orofacial region: Site and sex differences. Eur J Pain 23:81–90. https://doi.org/10.1002/ejp.1287
Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B (2006) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 123:231–243. https://doi.org/10.1016/j.pain.2006.01.041
Yarnitsky D (2015) Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 156(Suppl 1):S24–S31. https://doi.org/10.1097/01.j.pain.0000460343.46847.58
Moana-Filho EJ, Herrero Babiloni A (2019) Endogenous pain modulation in chronic temporomandibular disorders: Derivation of pain modulation profiles and assessment of its relationship with clinical characteristics. J Oral Rehabil 46:219–232. https://doi.org/10.1111/joor.12745
Oono Y, Wang K, Baad-Hansen L, Futarmal S, Kohase H, Svensson P, Arendt-Nielsen L (2014) Conditioned pain modulation in temporomandibular disorders (TMD) pain patients. Exp Brain Res 232:3111–3119. https://doi.org/10.1007/s00221-014-3997-7
Maixner W, Fillingim R, Booker D, Sigurdsson A (1995) Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain 63(3):341–351. https://doi.org/10.1016/0304-3959(95)00068-2
King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL (2009) Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain 143:172–178. https://doi.org/10.1016/j.pain.2008.12.027
Auerbach SM, Laskin DM, Frantsve LME, Orr T (2001) Depression, pain, exposure to stressful life events, and long-term outcomes in temporomandibular disorder patients. J Oral Maxillofac Surg 59:628–633. https://doi.org/10.1053/joms.2001.23371
Bonjardim LR, Gavião MBD, Pereira LJ, Castelo PM (2005) Anxiety and depression in adolescents and their relationship with signs and symptoms of temporomandibular disorders. Int J Prosthodont 18:347–352
Ekici Ö (2020) Association of stress, anxiety, and depression levels with sleep quality in patients with temporomandibular disorders. Cranio - J Craniomandibular Pract. https://doi.org/10.1080/08869634.2020.1861886
Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, MacK N, Slade GD, Maixner W (2013) Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J Pain 14. https://doi.org/10.1016/j.jpain.2013.06.009
Fillingim RB, Slade GD, Greenspan JD, Dubner R, Maixner W, Bair E, Ohrbach R (2018) Long-term changes in biopsychosocial characteristics related to temporomandibular disorder: Findings from the OPPERA study. Pain 159:2403–2413. https://doi.org/10.1097/j.pain.0000000000001348
Bair E, Gaynor S, Slade GD, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Smith SB, Diatchenko L, Maixner W (2016) Identification of clusters of individuals relevant to temporomandibular disorders and other chronic pain conditions: The OPPERA study. Pain 157:1266–1278. https://doi.org/10.1097/j.pain.0000000000000518
Meng H, Dai J, Li Y (2021) Quantitative sensory testing in patients with the muscle pain subtype of temporomandibular disorder: a systemic review and meta-analysis. Clin Oral Investig 25:6547–6559
Kothari S, Baad-Hansen L, Svensson P (2017) Psychosocial Profiles of Temporomandibular Disorder Pain Patients: Proposal of a New Approach to Present Complex Data. J Oral Facial Pain Headache 31:199–209. https://doi.org/10.11607/ofph.1666
Park JW, Clark GT, Kim YK, Chung JW (2010) Analysis of thermal pain sensitivity and psychological profiles in different subgroups of TMD patients. Int J Oral Maxillofac Surg 39:968–974. https://doi.org/10.1016/j.ijom.2010.06.003
Mason KJ, O’Neill TW, Lunt M, Jones AKP, McBeth J (2018) Psychosocial factors partially mediate the relationship between mechanical hyperalgesia and self-reported pain. Scand J Pain 18:59–69. https://doi.org/10.1515/sjpain-2017-0109
Welte-Jzyk C, Pfau DB, Hartmann A, Daubländer M (2018) Somatosensory profiles of patients with chronic myogenic temporomandibular disorders in relation to their painDETECT score. BMC Oral Health 18. https://doi.org/10.1186/s12903-018-0601-8
Nahit ES, Pritchard CM, Cherry NM, Silman AJ, Macfarlane GJ (2001) The influence of work related psychosocial factors and psychological distress on regional musculoskeletal pain: a study of newly employed workers. J Rheumatol 28(6):1378–1384
Ocay DD, Larche CL, Betinjane N, Jolicoeur A, Beaulieu MJ, Saran N, Ouellet JA, Ingelmo PM, Ferland CE (2022) Phenotyping Chronic Musculoskeletal Pain in Male and Female Adolescents: Psychosocial Profiles, Somatosensory Profiles and Pain Modulatory Profiles. J Pain Res 15:591–612. https://doi.org/10.2147/JPR.S352607
Ohrbach R (ed) (2016) Diagnostic Criteria for Temporomandibular Disorders: Assessment Instruments. Version 15May2016. [Critérios de Diagnóstico para Desordens Temporomandibulares: Protocolo Clínico e Instrumentos de Avaliação: Brazilian Portuguese Version 25May2016] Pereira Jr. FJ, Gonçalves DAG, Trans. www.rdc-tmdinternational.org
Sehn F, Chachamovich E, Vidor LP, Dall-Agnol L, Custódio de Souza IC, Torres ILS, Fregni F, Caumo W (2012) Cross-Cultural Adaptation and Validation of the Brazilian Portuguese Version of the Pain Catastrophizing Scale. Pain Med 13:1425–1435. https://doi.org/10.1111/j.1526-4637.2012.01492.x
Cohen S, Kamarck T, Mermelstein R (1983) A Global Measure of Perceived Stress. J Health Soc Behav 24:385–396
Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD (2006) Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur J Pain 10:77–88. https://doi.org/10.1016/j.ejpain.2005.02.003
Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, Yarnitsky D (2008) Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain 136:142–149. https://doi.org/10.1016/j.pain.2007.06.029
Nir RR, Granovsky Y, Yarnitsky D, Sprecher E, Granot M (2011) A psychophysical study of endogenous analgesia: The role of the conditioning pain in the induction and magnitude of conditioned pain modulation. Eur J Pain 15:491–497. https://doi.org/10.1016/j.ejpain.2010.10.001
Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O (2010) Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 14:339. https://doi.org/10.1016/j.ejpain.2010.02.004
Derogatis L (1986) SCL 90 R administration, scoring and procedures manual II for the revised version and other instruments of the psychopathology rating scale series. Clin Psychometric Res
Bair E, Tibshirani R (2004) Semi-supervised methods to predict patient survival from gene expression data. PLoS Biol 2. https://doi.org/10.1371/journal.pbio.0020108
Nielsen F (2016) Hierarchical clustering. In: Introduction to HPC with MPI for data science. Undergraduate Topics in Computer Science. Springer, Cham. https://doi.org/10.1007/978-3-319-21903-5_8
Slade GD, Diatchenko L, Bhalang K, Sigurdsson A, Fillingim RB, Belfer I, Max MB, Goldman D, Maixner W (2007) Influence of Psychological Factors on Risk of Temporomandibular Disorders. J Dent Res 86:1120–1125. https://doi.org/10.1177/154405910708601119
Pfau DB, Rolke R, Nickel R, Treede RD, Daublaender M (2009) Somatosensory profiles in subgroups of patients with myogenic temporomandibular disorders and fibromyalgia syndrome. Pain 147:72–83. https://doi.org/10.1016/j.pain.2009.08.010
Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Diatchenko L, Liu Q, Maixner W (2013) Pain sensitivity and autonomic factors associated with development of TMD: The OPPERA prospective cohort study. Journal of Pain 14. https://doi.org/10.1016/j.jpain.2013.06.007
Pillai RS, Pigg M, List T, Karlsson P, Mladenović Ž, Vase L, Nørholt SE, Pedersen TK, Bengtsson M, Costa YM, Svensson P, Baad-Hansen L (2020) Assessment of Somatosensory and Psychosocial Function of Patients with Trigeminal Nerve Damage. Clin J Pain 36:321–335. https://doi.org/10.1097/AJP.0000000000000806
Melzack R, Casey KL (1968) Sensory, motivational, and central control determinants of pain: a new conceptual model. The skin senses 1:423–43
Price DD (2000) Psychological and neural mechanisms of the affective dimension of pain. Science (New York) 288(5472):1769–1772. https://doi.org/10.1126/science.288.5472.1769
Talbot K, Madden VJ, Jones SL, Moseley GL (2019) The sensory and affective components of pain: are they differentially modifiable dimensions or inseparable aspects of a unitary experience? A systematic review. Br J Anaesth 123:e263–e272
Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(3 Suppl):S2–S15. https://doi.org/10.1016/j.pain.2010.09.030
Corrêa JB, Costa LOP, de Oliveira NTB, Sluka KA, Liebano RE (2015) Central sensitization and changes in conditioned pain modulation in people with chronic nonspecific low back pain: a case–control study. Exp Brain Res 233:2391–2399. https://doi.org/10.1007/s00221-015-4309-6
Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W (2011) Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. Journal of Pain 12. https://doi.org/10.1016/j.jpain.2011.08.006
La Touche R, Paris-Alemany A, Hidalgo-Pérez A, López-de-Uralde-Villanueva I, Angulo-Diaz-Parreño S, Muñoz-García D (2018) Evidence for central sensitization in patients with temporomandibular disorders: a systematic review and meta-analysis of observational studies. Pain Pract 18:388–409
Fernández-de-las-Peñas C, Galán-del-Río F, Fernández-Carnero J, Pesquera J, Arendt-Nielsen L, Svensson P (2009) Bilateral Widespread Mechanical Pain Sensitivity in Women With Myofascial Temporomandibular Disorder: Evidence of Impairment in Central Nociceptive Processing. Journal of Pain 10:1170–1178. https://doi.org/10.1016/j.jpain.2009.04.017
Svensson P, List T (2001) Analysis of stimulus-evoked pain in patients with myofascial temporomandibular pain disorders. Pain 92(3):399–409. https://doi.org/10.1016/S0304-3959(01)00284-6
Ayesh EE, Jensen TS, Svensson P (2007) Hypersensitivity to mechanical and intra-articular electrical stimuli in persons with painful temporomandibular joints. J Dent Res 86:1187–1192. https://doi.org/10.1177/154405910708601209
Vale Braido do GV, Svensson P, Santos Proença dos J, Mercante FG, Fernandes G, de GodoiGonçalves DA (2023) Are central sensitization symptoms and psychosocial alterations interfering in the association between painful TMD, migraine, and headache attributed to TMD? Clin Oral Investig 27:681–690. https://doi.org/10.1007/s00784-022-04783-5
Svensson P, Arendt-Nielsen L, Nielsen H, Larsen JK (1995) Effect of chronic and experimental jaw muscle pain on pain-pressure thresholds and stimulus-response curves. J Orofac Pain 9:347–356
Suvinen TI, Reade PC, Kemppainen P, Könönen M, Dworkin SF (2005) Review of aetiological concepts of temporomandibular pain disorders: Towards a biopsychosocial model for integration of physical disorder factors with psychological and psychosocial illness impact factors. Eur J Pain 9:613. https://doi.org/10.1016/j.ejpain.2005.01.012
De La Torre CG, Câmara-Souza MB, Muñoz Lora VRM, Guarda-Nardini L, Conti PCR, Rodrigues Garcia RM, Del Bel Cury AA, Manfredini D (2018) Prevalence of psychosocial impairment in temporomandibular disorder patients: A systematic review. J Oral Rehabil 45:881–889
Lawn T, Sendel M, Baron R, Vollert J (2023) Beyond biopsychosocial: the keystone mechanism theory of pain. Brain Behav Immun 114:187–192. https://doi.org/10.1016/j.bbi.2023.08.018
Acknowledgements
grant 2022/13506-9, São Paulo Research Foundation (FAPESP).
Funding
Funding sources: grant 2022/13506-9, São Paulo Research Foundation (FAPESP).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Rafaela Stocker Salbego, Flávia Fonseca Carvalho Soares, Dyna Mara Araújo Oliveira Ferreira, Matheus Herreira-Ferreira, Beatriz Lima Netto, Yuri Martins Costa, Paulo César Rodrigues Conti, Peter Svensson and Leonardo Rigoldi Bonjardim. The first draft of the manuscript was written by Rafaela Stocker Salbego and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript for this submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was submitted and approved by the Research Ethics Committee of the Bauru School of Dentistry, University of São Paulo (CAAE 63551122.7.0000.5417). All individuals who agreed to participate in the primary study signed an informed consent form.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the data used in this study.
Conflicts of interest
All authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salbego, R.S., Conti, P.C.R., Soares, F.F.C. et al. Influence of psychometric and sleep quality features on painful mechanical sensitivity and pain modulation in patients with temporomandibular disorders. Clin Oral Invest 28, 302 (2024). https://doi.org/10.1007/s00784-024-05699-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05699-y