Abstract
Objectives
We aimed to explore the osteogenic potential of periodontal ligament stem cells (PDLSCs) in bioprinted methacrylate gelatine (GelMA) hydrogels in vitro and in vivo.
Materials and methods
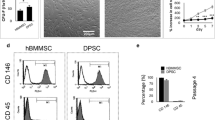
PDLSCs in GelMA hydrogels at various concentrations (3%, 5%, and 10%) were bioprinted. The mechanical properties (stiffness, nanostructure, swelling, and degradation properties) of bioprinted constructs and the biological properties (cell viability, proliferation, spreading, osteogenic differentiation, and cell survival in vivo) of PDLSCs in bioprinted constructs were evaluated. Then, the effect of bioprinted constructs on bone regeneration was investigated using a mouse cranial defect model.
Results
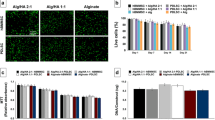
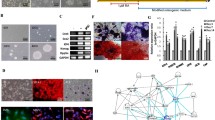
Ten percent GelMA printed constructs had a higher compression modulus, smaller porosity, lower swelling rate, and lower degradation rate than 3% GelMA. PDLSCs in bioprinted 10% GelMA bioprinted constructs showed lower cell viability, less cell spreading, upregulated osteogenic differentiation in vitro, and lower cell survival in vivo. Moreover, upregulated expression of ephrinB2 and EphB4 protein and their phosphorylated forms were found in PDLSCs in 10% GelMA bioprinted constructs, and inhibition of eprhinB2/EphB4 signalling reversed the enhanced osteogenic differentiation of PDLSCs in 10% GelMA. The in vivo experiment showed that 10% GelMA bioprinted constructs with PDLSCs contributed to more new bone formation than 10% GelMA constructs without PDLSCs and constructs with lower GelMA concentrations.
Conclusions
Bioprinted PDLSCs with high-concentrated GelMA hydrogels exhibited enhanced osteogenic differentiation partially through upregulated ephrinB2/EphB4 signalling in vitro and promoted bone regeneration in vivo, which might be more appropriate for future bone regeneration applications.
Clinical relevance
Bone defects are a common clinical oral problem. Our results provide a promising strategy for bone regeneration through bioprinting PDLSCs in GelMA hydrogels.










Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Genova T, Roato I, Carossa M, Motta C, Cavagnetto D, Mussano F (2020) Advances on bone substitutes through 3D bioprinting. Int J Mol Sci 21. https://doi.org/10.3390/ijms21197012
Tournier P, Guicheux J, Pare A, Maltezeanu A, Blondy T, Veziers J, Vignes C, Andre M, Lesoeur J, Barbeito A, Bardonnet R, Blanquart C, Corre P, Geoffroy V, Weiss P, Gaudin A (2021) A partially demineralized allogeneic bone graft: in vitro osteogenic potential and preclinical evaluation in two different intramembranous bone healing models. Sci Rep 11:4907. https://doi.org/10.1038/s41598-021-84039-6
Piard C, Baker H, Kamalitdinov T, Fisher J (2019) Bioprinted osteon-like scaffolds enhance in vivo neovascularization. Biofabrication 11:025013. https://doi.org/10.1088/1758-5090/ab078a
Du L, Qin C, Zhang H, Han F, Xue J, Wang Y, Wu J, Xiao Y, Huan Z and Wu C (2023) Multicellular bioprinting of biomimetic inks for tendon-to-bone regeneration. Adv Sci (Weinh) e2301309. https://doi.org/10.1002/advs.202301309
Madl CM, Heilshorn SC, Blau HM (2018) Bioengineering strategies to accelerate stem cell therapeutics. Nature 557:335–342. https://doi.org/10.1038/s41586-018-0089-z
Yang Y, Wang M, Yang S, Lin Y, Zhou Q, Li H, Tang T (2020) Bioprinting of an osteocyte network for biomimetic mineralization. Biofabrication 12:045013. https://doi.org/10.1088/1758-5090/aba1d0
Heo DN, Ayan B, Dey M, Banerjee D, Wee H, Lewis GS, Ozbolat IT (2020) Aspiration-assisted bioprinting of co-cultured osteogenic spheroids for bone tissue engineering. Biofabrication 13. https://doi.org/10.1088/1758-5090/abc1bf
Ratheesh G, Vaquette C, Xiao Y (2020) Patient-specific bone particles bioprinting for bone tissue engineering. Adv Healthc Mater e2001323. https://doi.org/10.1002/adhm.202001323
Wang T, Li W, Zhang Y, Xu X, Qiang L, Miao W, Yue X, Jiao X, Zhou X, Ma Z, Li S, Ding M, Zhu J, Yang C, Wang H, Li T, Sun X, Wang J (2023) Bioprinted constructs that simulate nerve-bone crosstalk to improve microenvironment for bone repair. Bioact Mater 27:377–393. https://doi.org/10.1016/j.bioactmat.2023.02.013
Hu X, Li H, Qiao L, Yang S, Wu H, Peng C, Zhang Y, Lan H, Yang H and Li K (2023) Fabrication of 3D gel-printed β-tricalcium phosphate/titanium dioxide porous scaffolds for cancellous bone tissue engineering. Int J Bioprinting 9. https://doi.org/10.18063/ijb.v9i2.673
Yang X, Ma Y, Wang X, Yuan S, Huo F, Yi G, Zhang J, Yang B, Tian W (2023) A 3D-bioprinted functional module based on decellularized extracellular matrix bioink for periodontal regeneration. Adv Sci (Weinh) 10:e2205041. https://doi.org/10.1002/advs.202205041
Hao Y, Cao B, Deng L, Li J, Ran Z, Wu J, Pang B, Tan J, Luo D, Wu W (2023) The first 3D-bioprinted personalized active bone to repair bone defects: a case report. Int J Bioprint 9:654. https://doi.org/10.18063/ijb.v9i2.654
Yamada Y, Ito K, Nakamura S, Ueda M, Nagasaka T (2011) Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transplant 20:1003–1013. https://doi.org/10.3727/096368910X539128
Ma Y, Ji Y, Zhong T, Wan W, Yang Q, Li A, Zhang X, Lin M (2017) Bioprinting-based PDLSC-ECM screening for in vivo repair of alveolar bone defect using cell-laden, injectable and photocrosslinkable hydrogels. ACS Biomater Sci Eng 3:3534–3545. https://doi.org/10.1021/acsbiomaterials.7b00601
Yan C, Li N, Xiao T, Ye X, Fu L, Ye Y, Xu T, Yu J (2022) Extracellular vesicles from the inflammatory microenvironment regulate the osteogenic and odontogenic differentiation of periodontal ligament stem cells by miR-758-5p/LMBR1/BMP2/4 axis. J Transl Med 20:208. https://doi.org/10.1186/s12967-022-03412-9
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155. https://doi.org/10.1016/S0140-6736(04)16627-0
Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou J, Jin Y, Chen FM (2013) Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials 34:7033–7047. https://doi.org/10.1016/j.biomaterials.2013.05.025
Thattaruparambil Raveendran N, Vaquette C, Meinert C, Samuel Ipe D, Ivanovski S (2019) Optimization of 3D bioprinting of periodontal ligament cells. Dent Mater 35:1683–1694. https://doi.org/10.1016/j.dental.2019.08.114
Liu W, Heinrich MA, Zhou Y, Akpek A, Hu N, Liu X, Guan X, Zhong Z, Jin X, Khademhosseini A, Zhang YS (2017) Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv Healthc Mater 6. https://doi.org/10.1002/adhm.201601451
Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P (2014) The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 35:49–62. https://doi.org/10.1016/j.biomaterials.2013.09.078
Pepelanova I, Kruppa K, Scheper T, Lavrentieva A (2018) Gelatin-Methacryloyl (GelMA) Hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering (Basel) 5. https://doi.org/10.3390/bioengineering5030055
Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A (2010) Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31:5536–5544. https://doi.org/10.1016/j.biomaterials.2010.03.064
Xie M, Zheng Y, Gao Q, He Y (2020) Facile 3D cell culture protocol based on photocurable hydrogels. Bio-Design Manuf 4:149–153. https://doi.org/10.1007/s42242-020-00096-2
Zonderland J, Moroni L (2021) Steering cell behavior through mechanobiology in 3D: a regenerative medicine perspective. Biomaterials 268:120572. https://doi.org/10.1016/j.biomaterials.2020.120572
Kim C, Young JL, Holle AW, Jeong K, Major LG, Jeong JH, Aman ZM, Han DW, Hwang Y, Spatz JP, Choi YS (2020) Stem cell mechanosensation on gelatin methacryloyl (GelMA) stiffness gradient hydrogels. Ann Biomed Eng 48:893–902. https://doi.org/10.1007/s10439-019-02428-5
Lee HP, Gu L, Mooney DJ, Levenston ME, Chaudhuri O (2017) Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat Mater 16:1243–1251. https://doi.org/10.1038/nmat4993
Liu N, Zhou M, Zhang Q, Yong L, Zhang T, Tian T, Ma Q, Lin S, Zhu B, Cai X (2018) Effect of substrate stiffness on proliferation and differentiation of periodontal ligament stem cells. Cell Prolif 51:e12478. https://doi.org/10.1111/cpr.12478
Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K (2006) Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 4:111–121. https://doi.org/10.1016/j.cmet.2006.05.012
Toda H, Yamamoto M, Uyama H, Tabata Y (2017) Effect of hydrogel elasticity and ephrinB2-immobilized manner on Runx2 expression of human mesenchymal stem cells. Acta Biomater 58:312–322. https://doi.org/10.1016/j.actbio.2017.03.016
Wang P, Wang W, Geng T, Liu Y, Zhu S, Liu Z, Yuan C (2019) EphrinB2 regulates osteogenic differentiation of periodontal ligament stem cells and alveolar bone defect regeneration in beagles. J Tissue Eng 10:2041731419894361. https://doi.org/10.1177/2041731419894361
Diercke K, Kohl A, Lux CJ, Erber R (2011) Strain-dependent up-regulation of ephrin-B2 protein in periodontal ligament fibroblasts contributes to osteogenesis during tooth movement. J Biol Chem 286:37651–37664. https://doi.org/10.1074/jbc.M110.166900
Ouyang L, Yao R, Zhao Y, Sun W (2016) Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 8:035020. https://doi.org/10.1088/1758-5090/8/3/035020
Gonzalez-Fernandez T, Tenorio AJ, Campbell KT, Silva EA, Leach JK (2021) Alginate-based bioinks for 3D bioprinting and fabrication of anatomically accurate bone grafts. Tissue Eng Part A 27:1168–1181. https://doi.org/10.1089/ten.TEA.2020.0305
Moghanian A, Portillo-Lara R, Shirzaei Sani E, Konisky H, Bassir SH, Annabi N (2020) Synthesis and characterization of osteoinductive visible light-activated adhesive composites with antimicrobial properties. J Tissue Eng Regen Med 14:66–81. https://doi.org/10.1002/term.2964
Roato I, Masante B, Putame G, Massai D, Mussano F (2022) Challenges of periodontal tissue engineering: increasing biomimicry through 3D printing and controlled dynamic environment. Nanomaterials (Basel) 12. https://doi.org/10.3390/nano12213878
Celikkin N, Mastrogiacomo S, Jaroszewicz J, Walboomers XF, Swieszkowski W (2018) Gelatin methacrylate scaffold for bone tissue engineering: the influence of polymer concentration. J Biomed Mater Res A 106:201–209. https://doi.org/10.1002/jbm.a.36226
Hannink G, Arts JJ (2011) Bioresorbability, porosity and mechanical strength of bone substitutes: what is optimal for bone regeneration? Injury 42(Suppl 2):S22–S25. https://doi.org/10.1016/j.injury.2011.06.008
Lee HP, Stowers R, Chaudhuri O (2019) Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nat Commun 10:529. https://doi.org/10.1038/s41467-019-08465-x
Kumar G, Waters MS, Farooque TM, Young MF, Simon CG Jr (2012) Freeform fabricated scaffolds with roughened struts that enhance both stem cell proliferation and differentiation by controlling cell shape. Biomaterials 33:4022–4030. https://doi.org/10.1016/j.biomaterials.2012.02.048
Zheng L, Yang F, Shen H, Hu X, Mochizuki C, Sato M, Wang S, Zhang Y (2011) The effect of composition of calcium phosphate composite scaffolds on the formation of tooth tissue from human dental pulp stem cells. Biomaterials 32:7053–7059. https://doi.org/10.1016/j.biomaterials.2011.06.004
Wei W, Liu W, Kang H, Zhang X, Yu R, Liu J, Huang K, Zhang Y, Xie M, Hu Y, Dai H (2023) A one-stone-two-birds strategy for osteochondral regeneration based on a 3D printable biomimetic scaffold with kartogenin biochemical stimuli gradient. Adv Healthc Mater e2300108. https://doi.org/10.1002/adhm.202300108
Shi Z, Zhong Q, Chen Y, Gao J, Pan X, Lian Q, Chen R, Wang P, Wang J, Shi Z, Cheng H (2021) Nanohydroxyapatite, nanosilicate-reinforced injectable, and biomimetic gelatin-methacryloyl hydrogel for bone tissue engineering. Int J Nanomedicine 16:5603–5619. https://doi.org/10.2147/IJN.S321387
Pu X, Tong L, Wang X, Liu Q, Chen M, Li X, Lu G, Lan W, Li Q, Liang J, Sun Y, Fan Y, Zhang X (2022) Bioinspired hydrogel anchoring 3DP GelMA/HAp scaffolds accelerates bone reconstruction. ACS Appl Mater Interfaces 14:20591–20602. https://doi.org/10.1021/acsami.1c25015
Acknowledgements
The authors thank the Public Experimental Center of Xuzhou Medical University for their help and support.
Funding
This study was supported by National Nature Science Foundation of China (grant no. 82201071), Xuzhou Key Research and Development Project (grant no. KC21236), Research Program of the Jiangsu Provincial Health Commission (grant no. M2020091), and Innovation and Entrepreneurship Training Program for College Students of Jiangsu Province (202110313009Z).
Author information
Authors and Affiliations
Contributions
Y.Z. and W.W. contributed to conceptualization (lead); writing—original draft (lead); formal analysis (lead); writing— review and editing (equal). Q.C. and T.R. assisted with methodology (lead); writing—review and editing (equal). J.Y. and G.L. and Y.Q. provided software (lead); writing—review and editing (equal). C.Y. contributed to conceptualization (supporting); writing—review and editing (lead). P.W. assisted with conceptualization (supporting); writing—original draft (supporting); writing—review and editing (equal).
Corresponding authors
Ethics declarations
Ethical approval
Human third molars were obtained from healthy volunteers with their written informed consent. Animal experiments in this study were approved by the Experimental Animal Ethics Committee of Xuzhou Medical University, approval No.202208S107. All experimental procedures were performed in accordance with the corresponding guidelines and regulations. All animals were kept in a pathogen-free environment and there were no incidents of animal cruelty.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaru Zhu and Wen Wang contributed equally to this work and share first authorship.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Wang, W., Chen, Q. et al. Bioprinted PDLSCs with high-concentration GelMA hydrogels exhibit enhanced osteogenic differentiation in vitro and promote bone regeneration in vivo. Clin Oral Invest 27, 5153–5170 (2023). https://doi.org/10.1007/s00784-023-05135-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05135-7