Abstract
Objectives
To assess the outcome of leukocyte-platelet-rich fibrin (L-PRF) on the rate of maxillary canine retraction and its correlation with the levels of Receptor activator of nuclear factor kappa-B ligand (RANKL), osteoprotegerin (OPG), and RANKL:OPG in the gingival crevicular fluid (GCF) during comprehensive orthodontic treatment.
Subjects and methods
Eighteen females who required all 1st premolars extraction for the correction of their class I bimaxillary protrusion malocclusions were included. The L-PRF plugs were placed in the experimental side 1st premolar extraction sockets. Canine retraction was performed by sliding mechanics. Canine retraction was assessed from the maxillary study models prepared just before the extraction (T0) and then at 1 week (T1), 2 weeks (T2), 4 weeks (T3), and 8 weeks (T4) after the 1st premolar extraction and placement of L-PRF plugs. The concentrations of RANKL and OPG in the GCF were evaluated at T0, T1, T2, T3, and T4.
Results
In experimental sides, the amount of canine retraction was statistically more during the T0–T1, T1–T2, and T2–T3 periods. The mean concentration of RANKL at T1, T2, and T3 was significantly more in the experimental sides. The mean concentration of OPG was significantly less in the experimental sides at T2, T3, and T4. The RANKL:OPG was significantly more in the experimental sides at T1, T2, T3, and T4. No significant correlation was found between amount of canine retraction and concentration of RANKL and OPG and RANKL to OPG ratio in GCF.
Conclusions
The L-PRF accelerated the rate of maxillary canine retraction by 0.28 mm over an 8-week period. The L-PRF favored the local osteoclastogenesis by enhancing the RANKL and suppressing the OPG concentrations. There was no significant correlation between the rate of maxillary canine retraction and expression of RANKL, OPG, and RANKL:OPG in GCF.
Trial registration
The Clinical Trials Registry of India (Reg. No. CTRI/2020/10/028390, Date-13.10.2020).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orthodontic tooth movement involves iatrogenically induced controlled inflammation in the periodontal ligament and alveolar bone allowing subsequent healing process [1]. Following the application of orthodontic force, there is an alterations of blood circulation in the periodontal microvascular region [1]. The changes in the periodontal microvascular result release of various inflammatory mediators such as cytokines, interleukins, growth factors, colony-stimulating factors, arachidonic acid metabolites, and neurotransmitters [1]. These inflammatory mediators play a crucial role in the remodeling of periodontal ligament and alveolar bone and influence the rate of orthodontic tooth movement (OTM) [1]. The duration of comprehensive orthodontic treatment is usually 24–28 months in adults and primarily depends on the rate of OTM [2]. Long treatment duration results in many complications like development of white spot lesions, root resorption, gingivitis, and poor patient compliance [3]. Thus, various invasive and non-invasive modalities have been attempted to reduce the duration of orthodontic treatment by accelerating the rate of OTM.
Invasive modalities, like micro-osteoperforation [4], piezocision [5], piezopuncture [6], corticision [7], periodontally accelerated osteogenic orthodontics [8], dentoalveolar distraction [9], and periodontal ligament distraction [10] utilize the regional acceleratory phenomenon for increasing the rate of OTM. Although these invasive methods are termed as minimally invasive surgical procedures, they do carry side effects like decrease in alveolar bone density, alveolar bone resorption, poor status of periodontal health, and post-treatment root resorption [11]. Thus, to avoid various complications of invasive modalities, non-invasive modalities like the application of direct electric current stimulation [12, 13], vibration [14], low-dose laser therapy [15], and drugs like prostaglandin [16], relaxin [17, 18], and vitamin D injection [19] have been tried to accelerate OTM. However, the success of these non-invasive modalities is inconsistent [17, 18].
Currently, blood concentrates like platelet-rich plasma (PRP) [20, 21] and platelet-rich fibrin (PRF) [22,23,24,25,26,27] are used as non-invasive modality to accelerate the OTM. The leukocyte-platelet-rich fibrin (L-PRF) has been termed as the second-generation platelet concentrate. The L-PRF composed of a 3-dimensional fibrin matrix that traps a variety of blood cells. The L-PRF is enriched with autologous platelets, growth factors, cytokines, and leukocytes that direct the various cells in local tissue remodeling by promoting extracellular matrix synthesis, cell proliferation and differentiation, angiogenesis, and chemotaxis [28]. Receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG) are biomolecules of osteoblastic origin that regulate the osteoclastogenesis and bone resorption in both physiologic and pathologic conditions [29]. During the OTM, RANKL is expressed on the compressed side of the teeth and plays a crucial role in regulating bone remodeling [30]. The RANKL and its equivalent receptor RANK are protein ligands that share homology with the tumor necrosis factor (TNF) receptor superfamily. The interaction between RANKL and RANK is controlled by the soluble decoy receptor OPG, which is released by cells of the osteoblastic family and functions as a competitive inhibitor of RANKL [30]. The RANKL is present on the surface of osteoblasts, and it triggers osteoclastogenesis by binding to RANK, which is a transmembrane protein receptor located on osteoclast progenitors and osteoclasts [30]. Thus, the ratio of RANKL to OPG controls osteoclastogenesis. These RANKL and OPG are readily expressed in the gingival crevicular fluid (GCF) and can be correlated with the active OTM. Tehranchi et al. [22] and Nemtoi et al. [23] reported faster extraction space closure following the placement of L-PRF in the extraction socket. Karakasli et al. [24] noticed that injectable-platelet-rich fibrin (i-PRF) could increase the rate of maxillary incisor retraction and recommended its use to enhance the orthodontic tooth movement. However, Reyes et al. [25] observed decreased rate of canine retraction in young adults following L-PRF application, giving a contradictory opinion. Recently, Barhate et al. [26] reported very minimum beneficial effect of L-PRF on OTM. Thus, the present study was conducted to evaluate the efficacy of L-PRF on the rate of maxillary canine retraction and validate it by identifying levels of RANKL and OPG in the GCF.
Materials and methods
Trial design
The present study was a single-center, randomized controlled trial with a split-mouth design having 1:1 allocation ratio. The study was approved by the Institute Ethical Committee (IEC No. IEC/AIIMS BBSR/PG THESIS/2020-21/04). The study protocol was registered at The Clinical Trials Registry of India (Reg. No. CTRI/2020/10/028390).
Sample size
Assuming the mean difference of 1 mm (control side, 2 mm, and experimental side, 3 mm) in the total amount of tooth movement between the sides, 0.75 mm as standard deviation (SD), 95% power, 95% confidence interval, and 0.05 as alpha error, a sample size of 15 in. each side was calculated. Considering the 20% loss to follow-up, the sample size in each side was inflated to 18. Open Epi version 3, an open-source calculator, was used for sample size calculation.
Participants, eligibility, and settings
Participants were recruited from the Orthodontic Clinic, at the Dental Surgery OPD (January 2021 to March 2022) at All India Institute of Medical Sciences, Bhubaneswar, India. Eighteen female subjects of age within 18–25 years having class I bimaxillary dentoalveolar protrusion malocclusion and normo-divergent growth pattern (FMA, 20–28°) were screened initially to fulfil inclusion and exclusion criteria to enroll them in the study. All the subjects had minimum crowding or spacing (≤ 5 mm) in maxillary and mandibular arches, full complement of teeth except 3rd molars, and good oral hygiene (probing depth < 3 mm, gingival index score < 1). All subjects required 1st premolars extraction for the correction of their malocclusions.
Subjects who had history of comprehensive orthodontic treatment, failed to maintain good oral hygiene during the study period, were taking any medications for systemic illness, on NSAID, with metabolic disorder or syndrome affecting calcium and phosphate metabolism, known blood disorders like thrombocytopenia and leukopenia, and pregnant women were excluded from the study. An informed written consent was obtained from each eligible participant.
Randomization and blinding
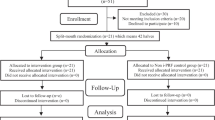
In each subject, the allocation of control and experimental sides (right vs. left) was done using a computer random number generator software (Microsoft Excel, Microsoft, Washington, USA). The patient allocation and their follow-up process during the study period are mentioned in Fig. 1. The outcome assessor was blinded during study model and biochemical assessment stages.
Intervention
The same orthodontist (BKV) provided orthodontic treatment for all subjects. Pre-welded bands were placed on first and second molars. Standard edgewise 0.018″ (American Orthodontics, WI, USA) brackets were used in all the subjects. Levelling and alignment of the maxillary arch were carried out followed by atraumatic extraction of 1st premolars under local anesthesia (2% lignocaine hydrochloride with adrenaline bitartrate (1:80,000)).
Prior to the extraction of maxillary 1st premolars, 10 ml of intravenous blood was collected in a plain centrifugation tube and centrifuged at a speed of 2700 rpm for 12 min [31]. After centrifugation, 3 segments were observed. The L-PRF plug was removed with the help of sterile tweezers and separated by dissecting it approximately 2 mm below the junction of the middle and lower layers with the help of no. 15 BP blade. The L-PRF plug was immediately placed on the experimental side 1st premolar extraction socket and secured in place with the help of 3–0 silk sutures. A sterile gauze pack was placed over the socket, and patient was asked to bite on it gently. On the control side, 1st premolar was extracted atraumatically with the help of extraction forceps, and a sterile gauze pack was placed over the socket. Same post-extraction instructions were given to all the subjects. For post-extraction pain, acetaminophen (500 mg) tablet was prescribed for moderate to severe pain.
Individual canine retraction was started on experimental and control sides by sliding mechanics on 0.016″ × 0.022″ stainless steel archwire by using the NiTi closed coil spring (0.9 mm × 12 mm), delivering a force of 150 g (Koden, Kozhikode, Kerala, India). The force exerted by NiTi-closed coil spring was measured with the Dontrix gauge (Dentmark, Ludhiana, India) and adjusted at each visit. The oral hygiene instructions were reinforced to each subject in every visit. No complications to any subject occurred during the trial.
Outcome measures
Model analysis
Canine retraction was assessed from the maxillary study models prepared just before the extraction (T0) and then at 1 week (T1), 2 weeks (T2), 4 weeks (T3), and 8 weeks (T4) after the 1st premolars extraction and placement of L-PRF plugs. Study models were scanned with Maestro 3D scanner (AGE solution, S.r.l, Italy) up to an accuracy of <8 μ to obtain the 3D model stereolithography (STL) file. The maxillary 3D model STL file was uploaded to Dolphin Imaging 11.95 Premium software (Dolphin Imaging & Management Solutions, Chatsworth, CA, USA) to assess maxillary canine retraction. A pair of 3D digital models (representing two particular time-points of the study) were loaded in the software, of which one was fixed, and the other was the moving model (Fig. 2A). The moving visualization surface of the 3D model was added to the baseline fixed model, and registration was done using the medial points of the third palatal rugae and the incisive papilla as reference landmarks (Fig. 2B) [32]. The best fit of the superimposition was evaluated by a color map with a spectrum of colors on the 3D digital models with two models used independently to determine the antero-posterior displacements of the canines (Fig. 2C) [32]. The amount of canine retraction on both sides was measured by marking the tips of canines in the pre- and post-models. The distance between the canine tips was evaluated 3-dimensionally, using the landmark plotting method, which was marked on X-axis at the tips of canines in pre and post-models. These were also checked in Y- and Z-axes to improve plotting reliability.
Collection of GCF
In both experimental and control sides, the GCF was collected from the distal side of gingival sulcus of maxillary canines at T0, T1, T2, T3, and T4 with the help of calibrated volumetric microcapillary pipettes having graduated markings (Accupipette T-10, Tarson, Kolkata, India). Adequate isolation was ensured with cotton rolls, and the sulcus was air dried by gentle strokes of compressed air. The microcapillary pipette was inserted gently at the gingival crevice and five microliters of GCF was collected as suggested by Griffiths (2000) [33]. Collected GCF was diluted by adding 245 µl of phosphate buffer solution (pH 7.4) and stored at −80 °C until biomarker assessment.
Assessment of biomarkers in GCF
The assessment of RANKL and OPG in the GCF was done by the enzyme-linked immunosorbent assay (ELISA). The kits of commercially available human RANKL and OPG ELISA kits (Cat. No. E0620Hu, BT lab, Shanghai, China) were used for the assessment according to the manufacturer’s guidelines. The minimum detection limit for RANKL and OPG was 0.075 ng/ml and 0.023 ng/ml, respectively. Duplicate standard points were prepared by serially diluting the standard solution. A standard of 50 µl was added to standard wells. A 40-µl sample was added into the sample wells. Then, 10 µl of anti-RANKL and anti-OPG antibodies was added to the sample wells. After that, 50 µl streptavidin-HRP conjugate was added to all standard and sample wells. The plate was then incubated for 60 min at 37 °C. After washing, 50 µl of substrate solutions was added to each well. Followed by the incubation for 10 min at 37 °C in the dark, stop solution was added and readings were taken at 450 nm using an ELISA microplate reader (Bio Tek, Winooski, USA). The standard curve was constructed. The concentrations of RANKL and OPG in GCF samples were calculated form the standard curve.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS, version-24, IBM, Chicago, IL) was used for the statistical analysis. The normality in the distribution of data was identified by using the Shapiro–Wilk test. Descriptive statistics, independent t-test analysis of variance (ANOVA), and Pearson correlation coefficient test were used. The P-value of 0.05 was considered as the level of statistical significance.
Results
Thirty-six extraction sockets in 18 subjects were randomly allocated to experimental and control sides of 18 each. Two subjects were lost to follow-up due to COVID-19 lockdown, and the remaining 16 subjects were followed till the end of study period. The CONSORT flow diagram is shown in Fig. 1.
At the beginning of study, the mean age of the subjects was 21.93 ± 1.73 years. The details of cephalometric dento-skeletal parameters at the beginning of orthodontic treatment and hematological parameters of the subjects at the time of L-PRF preparation are described in Table 1. The mean gingival index score of the subjects was 0.34 ± 0.11, 0.42 ± 0.12, 0.52 ± 0.11, 0.63 ± 0.16, and 0.68 ± 0.17 at T0, T1, T2, T3, and T4, respectively. The mean probing depth of the gingival sulcus at T0, T1, T2, T3, and T4 was 1.77 ± 0.41 mm, 1.98 ± 0.29 mm, 2.06 ± 0.57 mm, 2.10 ± 0.29 mm, and 2.10 ± 0.57 mm, respectively.
The details of canine retraction are described in Table 2. During the first (T0–T1) and second (T1–T2) weeks, the canine retraction was 0.06 mm (P < 0.05) and 0.12 mm (P < 0.01) more in the experimental sides. During next 2 weeks (T2–T3), there was only 0.04 mm more canine retraction in the experimental sides (P = 0.38). The amount of canine retraction during T3–T4 period was comparable between two sides (P = 0.204). During 8 weeks (T0–T4) of follow-up, the canine retraction was 0.28 mm more in the experimental sides (P < 0.01).
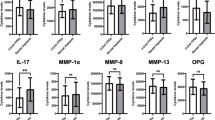
The change in the RANKL concentrations during study period is highlighted in Table 3. At baseline, the mean concentration of RANKL was comparable between two sides. At T1, the concentration increased by 2.80 ± 2.54 pg/μg and 9.55 ± 5.48 pg/μg in the control and experimental sides, respectively (P = 0.001). From T1 to T2, the concentration of RANKL increased marginally on both the sides. At T3, the concentration of RANKL decreased from its T2 value by 0.87 ± 2.70 pg/μg on the control side and 3.36 ± 3.01 pg/μg on the experimental side (P = 0.020). At T4, the concentration of RANKL decreased further to a level of 51.26 ± 3.39 pg/μg and 53.05 ± 2.60 pg/μg on the control and experimental sides, respectively.
The changes in the concentration of OPG at various time intervals of observation are shown in Table 3. At T0, the concentration of OPG was 92.44 ± 6.61 pg/μg and 93.28 ± 8.67 pg/μg on the control and experimental sides, respectively. The concentration decreased on both sides at T1, T2, T3, and T4, and the difference between the control and experimental sides was significant at T2 (P = 0.016), T3 (P = 0.020), and T4 (P = 0.023). At the beginning of study (T0), the RANKL:OPG ratio was 0.52 ± 0.05 and 0.53 ± 0.05 in. the control and experimental sides (Table 3). At T1, T2, T3, and T4, the ratio increased on both the sides being significantly more in the experimental sides (P < 0.01).
There was no statistically significant correlation between amount of canine retraction and the concentrations of RANKL, OPG, and RANKL to OPG ratio in the GCF (Table 4). There was a negative correlation between RANKL to OPG ratio with the amount of canine retraction in control sides, but the correlation was positive in experimental sides.
Discussion
Long treatment duration is a major concern for patients and orthodontists. Various invasive and non-invasive approaches have been tried to enhance the rate of OTM. Recently, blood concentrates like PRP and PRF have been used to accelerate OTM. However, studies evaluating the effect of L-PRF on the rate of tooth movement show contradictory results [22,23,24,25,26]. In the present study, we observed faster canine retraction during first 4 weeks following the L-PRF placement showing its beneficial effects on rate of OTM. We noticed a peak in the tooth movement during the second week of observation. The increased rate of tooth movement may be due to faster bone remodeling by the L-PRF. After 4 weeks, the L-PRF had no effect on the tooth movement. This could be due to the degradation of the L-PRF plug [34]. We observed 0.28 mm of extra tooth movement over a period of 8 weeks. Similar to our observation, Barhate et al. [26] reported 0.35 mm extra tooth movement over a period of 8 weeks. However, Tehranchi et al. [22] reported 0.38 mm of more tooth movement following the L-PRF placement over a 16-week period. In contrast to previous studies, Reyes et al. [6] noted a decreased rate of tooth movement following L-PRF placement. They explained that neo-angiogenesis and bone remodeling properties of L-PRF reduced rate of tooth movement.
The blood concentrates are a good source of various growth factors, thus play an important role in the cell proliferation and differentiation during tissue regeneration [35]. During orthodontic tooth movement, the bone homeostasis is maintained by the co-ordination between osteoblasts and osteoclasts. The application of orthodontic force leads to release of various cytokines. The cytokine interleukin-1β participates in the acute phase of cellular response to induce bone resorption through increased regulation by RANKL [36]. The RANKL facilitates the maturation of osteoclast precursors into mature osteoclasts, and this process is inhibited by osteoblastic production of OPG [36].
In the present study, we observed more expression of RANKL up to 8 weeks following the placement of L-PRF. The peak expression of RANKL was at 4 weeks. This increase could be due to the slow and sustained release of growth factors and pro-inflammatory cytokines from the L-PRF plug. The L-PRF plug helps retain the expressed RANKL in the local microenvironment where osteoclastogenesis is required. This process continues until L-PRF gets degraded in microenvironment [34]. Similar to our observation, Angel et al. also noted increased RANKL levels up to 42nd day following injection of PRP and 150 g force application for canine retraction [21]. We noted reduced expression of OPG during all the observation time points in both sides but the reduction was more marked in the L-PRF placement sides. From the second week of follow-up, there was a significant fall in the OPG levels in the experimental sides compared to the control sides, which continued till 8 weeks of observation. The decrease in concentration of OPG was maximum at 2 weeks on the L-PRF sides suggesting maximum suppression of osteoblastic activity. The late drop in the levels of OPG might be due to the anabolic effect of OPG, which is expressed after the catabolic effect of pro-inflammatory cytokines [37]. Similar to our observation, Angel et al. [21] also observed significantly less concentration of OPG at 7th and 30th days after injection of PRP. The ratio of RANKL to OPG at the distal sides of canines remained high on both sides till the end of 8 weeks, but it was significantly more on the L-PRF placement sides suggesting more bone resorption. At the end of 2 weeks, the ratio of RANKL to OPG was maximum indicating peak bone metabolism. Although the ratio of RANKL to OPG increased dramatically reflecting a rise in RANKL and indicating increased bone metabolism, we did not find any significant correlation between amount of tooth movement with the levels of RANKL and OPG in the GCF. However, the placement of L-PRF had a positive correlation between the amount of canine retraction and RANKL to OPG ratio. Kawasaki et al. [38] also did not find any significant correlation between levels of RANKL and OPG in the GCF with the speed of tooth movement. Another study by Barhate et al. [26] also did not find any significant correlation between rate of OTM and levels of interleukin-1β and tumor necrosis factor in GCF following the placement of L-PRF plugs.
Although a statistically significant increase in the rate of OTM was observed following L-PRF placement, it was not clinically effective in reducing the overall duration of comprehensive orthodontic treatment. Thus, L-PRF should be used with caution to accelerate OTM. The present study did not show any significant correlation between the rate of canine retraction and the concentration of RANKL and OPG in GCF. Further study should be planned to determine if any correlation exists between the rate of canine retraction and the expression of various biomarkers in GCF. A multi-centric study involving a wider number of subjects with a lower risk of selection and performance biases can be considered to achieve these objectives. Blinding of the investigator or the participants was not possible in the present study due to the nature of the intervention.
Conclusions
The following conclusions were drawn from the present study:
-
1.
The L-PRF accelerated the canine retraction by 0.28 mm over the period of 8 weeks.
-
2.
The L-PRF favored the local osteoclastogenesis by enhancing the RANKL and suppressing the OPG concentrations.
-
3.
There was no significant correlation between the amount of canine retraction and changes in the concentration of RANKL and OPG and RANKL to OPG ratio in the GCF following the placement of L-PRF.
Data availability
All the data will be available on request.
References
Reitan K (1960) Tissue behavior during orthodontic tooth movement. Am J Orthod 46:881–900
Fink DF, Smith RJ (1992) The duration of orthodontic treatment. Am J Orthod Dentofacial Orthop 102:45–51
Beckwith FR, Ackerman RJ Jr, Cobb CM, Tira DE (1999) An evaluation of factors affecting duration of orthodontic treatment. Am J Orthod Dentofacial Orthop 115:439–447
Alikhani M, Raptis M, Zoldan B, Sangsuwon C, Lee YB, Alyami B et al (2013) Effect of microosteoperforations on the rate of tooth movement. Am J Orthod Dentofacial Orthop 144:639–678
Diabart S, Sebaoun JD, Surmenian J (2009) Piezocision: a minimally invasive, periodontally accelerated orthodontic tooth movement procedure. Comped Contin Educ Dent 30:342–350
Kim YS, Kim SJ, Yoon HJ, Lee PJ, Moon W, Park YG (2013) Effect of piezopuncture on tooth movement and bone remodeling in dogs. Am J Orthod Dentofacial Orthop 144:23–31
Kim SJ, Park YO, Kang SG (2009) Effect of corticision on paradental remodeling in orthodontic tooth movement. Angle Orthod 79:284–291
Wilcko MW, Wilcko MT, Bouquot JE, Ferguson DJ (2001) Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodont Restor Dent 21:9–19
Sukurica Y, Karaman A, Gurel HG, Dolanmaz D (2007) Rapid canine distalization through segmental alveolar distraction osteogenesis. Angle Orthod 77:226–236
Liou EJ, Huang CS (1998) Rapid canine retraction through distraction periodontal ligament. Am J Orthod Dentofacial Orthop 114:372–382
Brezniak N, Wasserstein A (2002) Orthodontically induced inflammatory root resorption. Part-1: the basic science aspects. Angle Orthod 72:175–179
Davidovitch Z, Finkelson MD, Steigman S, Shanfeld JL, Montegomery PC, Korostoff E (1980) Electric currents, bone remodeling, and orthodontic tooth movement. I. The effect of electric currents on periodontal cyclic nucleotides. Am J Orthod 77:14–32
Davidovitch Z, Finkelson MD, Steigman S, Shanfeld JL, Montegomery PC, Korostoff E (1980) Electric currents, bone remodeling, and orthodontic tooth movement. II. Increase in rate of tooth movement and periodontal cyclic nucleotide levels by combined force and electric current. Am J Orthod 77:33–47
Yadav S, Dobie T, Assefnia A, Gupta H, Kajajzic Z, Nanda R (2015) Effect of low frequency mechanical vibration on orthodontic tooth movement. Am J Orthod Dentofacial Orthop 148:440–449
Yoshida T, Yamaguchi M, Utsunomiya T, Kato M, Arai Y, Kaneda T et al (2009) Low energy laser irradiation accelerates the velocity of tooth movement via stimulation of the alveolar bone remodeling. Orthod Craniofac Res 12:289–298
Yamasaki K, Shibata Y, Imai S, Tani Y, Shibasaki Y, Fukuhara T (1984) Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am J Orthod 85:508–518
Madan MS, Liu ZJ, Gu GM, King GJ (2007) Effects of human relaxin on orthodontic tooth movement and periodontal ligaments in rats. Am J Orthod Dentofacial Orthop 131:e1-10
McGorray SP, Dolce C, Kramer S, Stewart D, Wheeler TT (2012) A randomized, placebo-controlled clinical trial on the effects of recombinant human relaxin on tooth movement and short-term stability. Am J Orthod Dentofacial Orthop 141:196–203
Collins MK, Sinclarir PM (1988) The local use of vitamin D to increase the rate of orthodontic tooth movement. Am J Orthod Dentofacial Orthop 94:278–284
Liou EJ (2016) The development of submucosal injection of platelet rich plasma for accelerating orthodontic tooth movement and preserving pressure side alveolar bone. APOS Trends Orthod 6:5–11
Angel SL, Samrit VD, Kharbanda OP, Duggal R, Kumar V, Chauhan SS et al (2022) Effects of submucosally administered platelet-rich plasma on the rate of tooth movement: a single-center, split-mouth, randomized controlled trial with clinical and biochemical analysis. Angle Orthod 92:73–79
Tehranchi A, Behnia H, Pourdanesh F, Behnia P, Pinto N, Younessian F (2018) The effect of autologous leukocyte platelet rich fibrin on the rate of orthodontic tooth movement: a prospective randomized clinical trial. Eur J Dent 12:350–357
Nemtoi A, Sirghe A, Nemtoi A, Haba D (2018) The effect of a plasma with platelet-rich fibrin in bone regeneration and rate of orthodontic tooth movement in adolescents. Rev Chim 69:327–330
Karakasli K, Erdur EA (2021) The effect of platelet-rich fibrin (PRF) on maxillary incisor retraction rate. Angle Orthod 91:213–219
Reyes P, Contreras N (2020) Distalization rate of canine in alveolus filled with leukocyte-platelet fibrin in adults: randomized controlled clinical split-mouth trial. Am J Orthod Dentofacial Orthop 158:176–182
Barhate UH, Duggal I, Mangaraj M, Sharan J, Duggal R, Jena AK (2022) Effects of autologous leukocyte-platelet rich fibrin (L-PRF) on the rate of maxillary canine retraction and various biomarkers in gingival crevicular fluid (GCF): a split mouth randomized controlled trial. Int Orthod 20:100681
Barhate UH, Mangaraj M, Jena AK, Sharan J (2021) Applications of platelet rich fibrin in dental surgery. A comprehensive literature review. Trends Biomater Artif Organs 35:203–213
Prakash S, Thakur A (2011) Platelet concentrates: past, present and future. J Maxillofac Oral Surg 10:45–49
Belibasakis GN, Bostanci N (2012) The RANKL-OPG system in clinical periodontology. J Clin Periodontol 39:239–248
Oshiro T, Shiotani A, Shibasaki Y, Sasaki T (2002) Osteoclast induction in periodontal tissue during experimental movement of incisors in osteoprotegerin -deficient mice. Anat Rec 266:218–225
Choukron J, Adda F, Schoeffler C, Vervelle A (2001) Une oppurtunit’eenparo-implantologie: le PRF. Implantodontie 42:55–62
Stucki S, Gkantidis N (2020) Assessment of techniques used for superimposition of maxillary and mandibular 3D surface models to evaluate tooth movement: a systematic review. Eur J Orthod 42:559–570
Griffith GS (2003) Formation, collection and significance of gingival crevice fluid. Periodontol 2000 31:32–42
Kubesch A, Barbeck M, Al-Maawi S, Orlowska A, Booms PF, Sader RA et al (2019) A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets 30:329–340
Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA (2009) Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 37:2259–2272
Jandinski J (1988) Osteoclast activating factor is now interleukin-1 beta: historical perspective and biological implications. J Oral Pathol 17:145–152
Alikhani M, Alansari S, Sangsuwon C, Nervina J, Teixeira C (2016) Biphasic theory of tooth movement: cytokine expression and rate of tooth movement. In Biology of orthodontic tooth movement. Springer 1:45–65
Kawasaki K, Takahashi T, Yamaguchi M, Kasai K (2006) Effects of aging on RANKL and OPG levels in gingival crevicular fluid during orthodontic tooth movement. Orthod Craniofac Res 9:137–142
Funding
The study was partially funded by the Indian Council of Medical Research, New Delhi, India (No.: 3/2/Dec-2020/PG-Thesis-HRD (50D)).
Author information
Authors and Affiliations
Contributions
Balaram Krishna V: clinical works, literature searching, statistical analyses, preparation of draft manuscript.
Duggal Isha: study model analysis, data collection, final manuscript review.
Sharan Jitendra: clinical work supervision, final manuscript review.
Mangaraj Manaswini: biochemical analysis, data collection, final manuscript review.
Duggal Ritu: study model analysis, data collection, final manuscript review.
Jena Ashok Kumar: concept, study design, study supervision, preparation of final manuscript, manuscript correspondence.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An informed written consent was obtained from each participant. The study was approved by the Institute Ethical Committee (IEC No. IEC/AIIMS BBSR/PG THESIS/2020–21/04). The study protocol was registered at The Clinical Trials Registry of India (Reg. No. CTRI/2020/10/028390).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishna V., B., Duggal, I., Sharan, J. et al. Effect of leukocyte-platelet-rich fibrin (L-PRF) on the rate of orthodontic tooth movement and expression of various biomarkers in gingival crevicular fluid. Clin Oral Invest 27, 2311–2319 (2023). https://doi.org/10.1007/s00784-023-05026-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05026-x