Abstract
Objective
This systematic review aims to assess the available literature on the clinical efficacy of hand versus power-driven instruments for subgingival instrumentation during surgical periodontal therapy (ST).
Materials and methods
A search of the literature was carried out on MEDLINE via Ovid, Embase, Web of Science, the Cochrane Database, LILACS, and Scopus. RCTs comparing the use of powered instruments (test) to hand scalers (control) for subgingival instrumentation in terms of changes in probing pocket depth (PPD) after surgical periodontal treatment were included and screened in duplicate. Descriptive synthesis of the data and risk of bias assessment were undertaken.
Results
Four RCTs met the inclusion criteria and were included in this systematic review. ST in all studies was performed by means of open flap debridement. Gracey curettes were the most commonly used hand instruments, while sonic and ultrasonic devices were used in the test group. Sites with initial PPD ≥ 6 mm had pocket reduction ranging from 2.93 to 4.89 mm in the control group and from 2.77 to 3.86 mm in the test group. All studies found no significant difference between the different types of instruments/devices in terms of PPD reduction.
Conclusions
Despite the limited number of studies, both manual and power-driven instruments appear to be effective in reducing PPD after surgical treatment of periodontitis.
Clinical relevance
Based on the findings of this systematic review, the clinician may make a decision whether to use manual or powered instruments during ST on a case-by-case basis and considering other factors, such as the risk of creating high concentrations of aerosols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is a chronic multifactorial inflammatory disease in which initiation and progression depend on a dysbiotic dental plaque biofilm [1,2,3]. It is characterized by a destruction of the tooth-supporting tissues including clinical attachment loss (CAL) and radiographic alveolar bone loss, associated with periodontal pocket and gingival bleeding, which may eventually lead to tooth loss [2, 4]. The treatment of periodontitis requires a stepwise approach that aims to stop disease progression, improve clinical parameters, and reduce tissue inflammation by effectively removing biofilm and calculus from the tooth surface [3, 5]. Therefore, the patient’s optimal oral hygiene associated with professional supra- and subgingival instrumentation (step 1 and step 2) [3] and control of risk factors are essential for achieving the endpoints of periodontal treatment, i.e., pocket closure, defined as probing pocket depth (PPD) ≤ 4 mm and absence of gingival inflammation (i.e., no bleeding on probing – BoP) [3, 5,6,7].
Although most of patients/sites presenting periodontal pockets will positively respond to steps 1 and 2 of periodontal treatment, some sites may still present active disease after initial treatment [7,8,9,10,11,12]. In these areas, presenting deep residual pockets (PPD ≥ 6 mm) and BoP, surgical periodontal therapy (step 3) with the purpose of gaining further access to the root surface [13] through different surgical procedures may be considered [3, 5]. The access to root surfaces and removal of biofilm and calculus in the subgingival environment may be completed either with manual (i.e., curettes) and/or power-driven (i.e., sonic/ultrasonic devices) instruments [3, 11]. Over the years, sonic and ultrasonic scalers have been modified and designed to have smaller and thinner tips, with longer working ends, providing easier access to deeper pockets in furcation and intrabony defects. Studies have shown that either hand or power-driven instruments, or their combination, are effective to improve periodontal condition and clinical parameters after non-surgical periodontal therapy (NSPT), with no superiority of one instrument over the other [5, 11]. Thus, the current S3-level treatment guidelines strongly recommend for subgingival instrumentation, during step 2 of treatment, the use of hand or power-driven instruments either alone or in combination and leave the choice of instruments to the experience and preference of the operator and patient, respectively [3, 5].
Nevertheless, a significant disadvantage of power-driven scalers is the production of contaminated aerosols [15]. As such, additional care is required to achieve and maintain good infection control when incorporating these instruments into the patient’s treatment. The emergence of the SARS-CoV-2 virus and COVID-19 pandemic has had a significant effect on the delivery of routine dental treatments, particularly periodontal care [15,16,17]. In response to the new challenges, professional organizations published clinical guidelines providing guidance on the provision of periodontal care and use of aerosol-generating procedures (AGP) for patients [18]. Due to uncertainties about the safety of dental procedures, guidelines initially advised that aerosol-generating procedures should be avoided or, if necessary, undertaken with droplet and contact precautions and personal protective equipment (PPE) [18].
Although previous studies have compared the efficacy of manual versus power-driven instruments, they essentially focused on the non-surgical treatment of periodontitis (step 2) [19]. Therefore, the aim of this systematic review was to summarize the available literature on the clinical efficacy of hand versus power-driven instruments for subgingival instrumentation during periodontal surgical therapy (step 3). In other words, we were interested to clarify whether different instruments or their combination could influence pocket reduction after periodontal surgical intervention. This review provides useful information in the attempt to consider different approaches and types of instruments during surgical therapy of periodontitis, which may contribute to creation and implementation of future guidelines and protocols for periodontal treatment. It also provides relevant evidence to support treatment in those cases requiring reduced exposition to AGPs.
Material and methods
The systematic review protocol was registered with the International Register of Systematic Reviews (PROSPERO; CRD42020192679), and it is in line with the Cochrane Handbook [20]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was adopted.
Focused question
In periodontal patients undergoing periodontal surgery, what is the efficacy of hand compared to power-driven instruments for subgingival instrumentation in terms of probing pocket depth (PPD)reduction?
Inclusion criteria
Based on the PICOS, the main criteria for considering studies for this review are as follows:
-
a.
Types of participants: adult (≥ 18 years old) patients clinically diagnosed with periodontitis, undergoing periodontal surgery
-
b.
Types of interventions (test group): studies evaluating the use of power/mechanically driven instruments/scalers (e.g., ultrasonic scalers, sonic scalers, and rotatory instruments) combined or not with the use of hand scalers for instrumentation during periodontal surgical treatment
-
c.
Comparison (control group): patients receiving subgingival instrumentation with the aid of hand instruments (e.g., manual scalers and curettes) during periodontal surgical therapy
-
d.
Outcomes: primary outcome: changes/reduction in probing pocket depth (PPD) defined as the distance from the gingival margin to the base of the pocket; secondary outcomes: (1) percentage of pocket closure, defined as PPD ≤ 4 mm and no BoP; (2) changes in clinical attachment level (CAL); (3) changes in bleeding on probing (BoP); (4) changes in plaque index (PI); (5) tooth survival; (6) gingival recession; (7) radiographic changes (bone level changes); (8) changes in microbiota; (9) changes in gingival crevicular fluid (GCF) biomarkers; (10) patient-reported outcomes (including adverse events as reported by authors); and (11) time needed to perform the procedure
-
e.
Types of studies: randomized controlled trials (RCTs) and controlled clinical trials (CCTs) both with a parallel-group or split-mouth design and a minimum follow-up of 3 months post-surgery
Retrospective studies, prospective controlled studies (non-randomized), case series, case reports, review papers, in vitro studies, studies on animal models, opinion articles, and conference abstracts were excluded.
Search strategy
A sensitive strategy was developed aiming to identify studies reporting the use of hand and power-driven instruments for subgingival instrumentation during periodontal surgical therapy. The search strategy included MeSH terms and free text terms related to the Population (disease AND surgery), the Intervention, and the Comparison investigated in this review, connected with the Boolean operator “AND” (details on the search strategy can be found in supplementary file, Appendix 1). Six main databases were searched: MEDLINE via Ovid, Embase, Web of Science, the Cochrane Database (including the Central Register of Controlled Trials (CENTER), LILACS, and Scopus. A database search was conducted in 2020 and updated in March 2022. In an attempt to include both published and unpublished data, a specific theses database (https://about.proquest.com/en/dissertations/) and clinicaltrials.gov were searched. A hand search was performed for papers published about this topic, in high impact factor journals (Journal of Clinical Periodontology, Journal of Periodontology, Journal of Dental Research, Journal of Periodontal Research, Journal of Investigative and Clinical Dentistry) in the last year. Grey literature was investigated in opensigle.inist.fr. Papers published in English, Portuguese, Spanish, Italian, Greek, and German were considered.
Data management and data extraction
A two-stage screening (titles and abstract first and then full text) was carried out independently by two reviewers (JP and NC) using Rayyan QCRI free web app (https://www.rayyan.ai/). Any disagreement was resolved by discussion, and if necessary, a third reviewer (EC) was consulted. Prior to the formal screening process, a calibration exercise was undertaken to pilot and refine the screening questions. Calculation and presentation of level of agreement at each of the two-stage screening was carried out using kappa statistics.
Data extraction was also performed in duplicate by two reviewers (JP, NC). When data were only presented in figures and authors were not able to contact study investigators, information was extracted from figures by using software (Plot Digitizer) in line with the Cochrane Handbook. For the primary outcome (PPD changes/reduction), mean values and standard deviations (SDs) were extracted. Secondary outcomes were extracted if they were presented in at least one of the papers included. Only clinical parameters measured at least 3 months after the surgical procedure were extracted.
Quality assessment and risk of bias
Quality assessment of all the studies included was conducted independently and in duplicate (JP, NC), as part of the data extraction process. Since all included studies were RCTs, the revised Cochrane risk-of-bias tool for randomized trials (RoB 2.0) (updated August 2019) was employed. The RoB 2.0 assessment has five domains as follows: (1) bias arising from the randomization process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in measurement of the outcome, and (5) bias in selection of the reported result. In case of any inconsistence of the data between reviewers, information was checked. After the domain-level judgement, an overall risk of bias judgement was addressed for each of the included studies: low risk of bias, some concerns, or high risk of bias. Data on sample size calculation were also extracted.
Data synthesis
The PPD values at the different time points were extracted from the studies meeting the inclusion and exclusion criteria. PPD changes over time were calculated by subtracting the follow-up values from baseline (T0 − T1) When the SDs of the primary and secondary outcomes were not present, they were extrapolated by the formula: SD = (∆*√n)/t_(n − 1) when a punctual p value was reported and the tn − 1 value was calculable. When the p value was not reported and/or was not a punctual value (e.g., < 0.01), the SD was estimated by the formula: Variance (Δ) = Variance (x months) + Variance (basal) – 2r * SD (x months) * SD (basal), where r is Pearson’s correlation coefficient between the two times and it was fixed at 0.2 (low correlation) to be conservative. Whenever the information was available, the unit of analysis (patient or tooth) was extracted. When not available, changes in CAL, BoP, and PI were also calculated as per description above.
Only descriptive statistics were presented since meta-analysis was not performed due to the heterogeneity of the included studies.
Results
Study selection
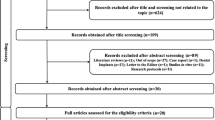
A total of 1295 records were identified and screened for the title and abstract, which led to 14 articles for full-text screening (Fig. 1). Only 4 studies met the inclusion criteria and were included in this systematic review. Reasons for exclusion of articles are described in Supplementary file (Appendix 2).
The level of agreement between reviewers was 93.3%, and Cohen’s kappa was 0.84 at full-text screening stage.
Study characteristics
The characteristics of the included studies are presented in Table 1. The total number of participants in each group (power-driven and hand instruments) was 50. Out of four randomized controlled trials included in this systematic review, 3 had a split-mouth design [21,22,23] and one a parallel-group design [14]. Studies included systemically healthy patients presenting periodontal disease defined as presence of moderate to advanced bone loss encompassing 40–60% of the root length [21, 22] or chronic periodontitis (PPD ≥ 5 mm) [14, 23]. In two studies, smokers were excluded while the remaining studies did not report data on smokers [14, 23]. All studies were carried out in a university/hospital setting, in Brazil [14, 23] and Germany [21, 22].
Sallum et al. [14] and Silva et al. [23] assessed anterior teeth while Kocher et al. [21, 22] evaluated single-rooted teeth and molars with furcation involvement. Only two studies specified the number of teeth/sites instrumented. In the study by Kocher et al. [21], 76 teeth (456 sites) were evaluated in the control group and 65 (390 sites) in the test group. Conversely, 45 molars with furcation involvement were investigated in a study performed by the same research group [22].
All studies included in this systematic review provided some type of initial therapy including oral hygiene instructions (OHI), plaque control, and/or supra/subgingival instrumentation (step 1 and step 2) before periodontal ST (step 3). In all studies, ST was performed by means of open flap debridement and was carried out by experienced operators, except in the study by Silva et al. [23] in which data on operator experience was not reported. In the study by Sallum et al. [14], surgeries were performed using a surgical microscope M-900 (DF Vasconcelos, São Paulo, Brazil).
Regarding the type of manual and power-driven instruments used, Gracey curettes were the most commonly used hand instruments, while sonic [21, 22] and ultrasonic [14, 23] devices were used in the test group. Three different power-driven instruments, primarily sonic/ultrasonic devices, were used in the test group (details for type of instrument are reported in Table 1). None of the studies investigated the combination of powered and hand instruments in the test group.
In the study by Sallum et al. [14], clinical measurement was performed with the aid of a stent, while in the other studies, no attempt of standardization was made. The follow-up varied from 3 to 24 months.
Primary outcome
Probing pocket depth (PPD) changes or reduction
The results demonstrated that both therapeutic approaches (hand or sonic/ultrasonic instrumentation) were effective in reducing PPD values from 3 to 24 months after surgical periodontal therapy [14, 21,22,23] (Table 2). On the experimental sites with an initial PPD of 4/5 mm, mean reduction in the studies included ranged from 0.86 to 1.82 mm in the control group and from 1.00 to 1.89 mm in the test group. Sites with initial PPD ≥ 6 mm had mean PPD reduction ranging from 2.93 to 4.89 mm in the control group and from 2.77 to 3.86 mm in the test group, respectively. However, no differences in the PPD reduction caused by the selection of instruments were shown in any of the studies [14, 21,22,23]. Kocher et al. [21, 22] also demonstrated no difference according to type of instruments in relation to the different tooth sites (with or without furcation; Table 2).
Secondary outcomes
Secondary outcomes are presented in Table 2.
Percentage of pocket closure (PPD ≤ 4 mm)
Percentage of pocket closure was not available in any of the studies.
Clinical attachment level (CAL)
In experimental sites with initial PPD ≤ 3 mm, an attachment loss ranging from 0.46 to 1.20 mm and 0.57 to 0.83 mm was observed over a period of 6 to 24 months in the control (hand instrument) and test group (ultrasonic/sonic scalers alone), respectively.
On the sites with an initial PPD ≥ 6 mm, CAL gain in the different studies ranged from 1.32 to 2.79 mm in the control group and from 0.55 to 2.11 mm in the test group. No difference between groups was found regardless of the instrumentation type [21, 23]. The same was observed in sites with furcation involvement [22].
In the study by Sallum et al. [14], CAL was described as the relative attachment level, i.e., the distance from a rubber stop to the most apical position of the periodontal pocket. However, the authors did not mention if the rubber stop was located at the cemento-enamel junction (CEJ). The findings from this study demonstrated a statistically significant difference in the relative attachment level between sites where calculus removal was performed with manual instruments (CDC) compared to ultrasonic scalers (CDUS). The differences were lower for the CDUS group (p < 0.0001).
Bleeding on probing
Gingival inflammation was measured in 3 studies as bleeding on probing (BoP) [21,22,23]. Root instrumentation during periodontal surgery with hand or power-driven scalers reduced BoP over time. However, the modality of root surface debridement had no influence on BoP at 6 and 24 months after the surgery around single-root teeth or [21] upper and lower molar sites [22].
Plaque accumulation
Plaque accumulation was recorded by dichotomous index [21, 22] on 3 sites per tooth and by Ainamo and Bay index [24] in the study by Silva et al. [23]. However, it was not clear if plaque was assessed only at experimental sites or on full mouth. In none of the studies, the modality of root surface debridement had influence on plaque accumulation 6 and 24 months after the surgery [21,22,23].
Gingival recession
Instrumentation with manual curettes or ultrasonic scaler with diamond-coated tips during periodontal surgery resulted in significant gingival recession compared to baseline. However, significant difference among groups was not found [23]. In the sites with initial PPD ≤ 3 mm, both instrument types showed an average mean recession of 0.5 mm that remained unchanged over the period of 24 months, regardless of the instrumentation modality [21]. It was not detailed in the studies if gingival recession was evaluated only at experimental sites or full mouth.
Time requirement
Of the 4 studies included in this review, only two recorded the time required for root surface instrumentation [21, 22]. In both studies, the time was recorded by stopwatch, not including changing of instruments or inspection of the root surface [21, 22]. When single-root teeth were instrumented with a sonic scaler, the average of time required was 1.4 ± 0.3 min, while with hand instruments, it was 3.0 ± 0.9 min (p < 0.001). Meanwhile, for instrumenting root surfaces of mandibular molars, 7.3 ± 1.0 min was necessary with manual instruments and 3.5 ± 1.5 min with sonic instruments (p < 0.01). For maxillary molars, 8.5 ± 1.5 min with hand instruments and 4.5 ± 1.4 min with diamond-coated sonic scaler inserts (p < 0.01) were required. Based on these findings, compared to hand instruments, the diamond-coated sonic scaler inserts markedly reduce the time required for root surface instrumentation of molars with furcation involvement and single-root teeth during flap surgery [21, 22].
Other secondary outcomes
Data on tooth survival, radiographic changes (bone level changes), changes in microbiota, gingival crevicular fluid (GCF) biomarkers, and patient-reported outcomes were not considered/investigated in any of the studies.
Assessment of methodological quality
Assessment of studies’ quality
All studies were considered at moderate risk due to insufficient reporting of the randomization process (namely lack of allocation concealment) and outcome measurement, while 25% of the studies had missing outcomes (Figs. 2 and 3). None of the studies reported if examiners were blinded and sample size calculation was not specified. In addition, the primary outcome was not clearly described in any of the studies.
Meta-analysis
Due to the significant heterogeneity in the study methodology (e.g., different time points) and reduced number of studies, a meta-analysis could not be performed.
Discussion
This systematic review summarizes the available evidence on the efficacy of hand and power-driven instruments for subgingival instrumentation during surgical treatment of periodontitis in terms of pocket reduction. Despite the limited amount of evidence available on this topic and the moderate risk of bias of the included studies, our results showed that irrespective of whether manual or power-driven instruments were used during surgical periodontal treatment, no difference was found in the clinical periodontal parameters. Even though only four studies were available on the present topic, all of them applied a similar surgical approach and were consistent in reporting similar outcomes for manual and powered instruments [14, 21,22,23]. Remarkably, while no changes in clinical outcomes were suggested, two studies indicated that powered instruments are more time-efficient, thus reducing the overall time of instrumentation [21, 22].
As part of the research efforts to support clinical practice, the findings of this systematic review generate relevant information when making decisions on the instruments to select for root instrumentation during periodontal surgery. In particular, the similar outcomes obtained with powered and manual instruments are extremely relevant in situations that do not allow use of power-driven instruments, such as when the risk of contamination due to the generation of contaminated aerosols in a closed environment is high, or in countries where access to ultrasonic and sonic devices may not be feasible. A recent systematic review has shown that ultrasonic scaling, air polishing, and prophylaxis procedures produce contamination, with a small amount of evidence showing droplets taking between 30 min and 1 h to settle [15]. In contrast, contamination associated with hand scaling was very low [15]. The emergence of the SARS-CoV-2 virus and COVID-19 pandemic has had a significant effect on the delivery of routine dental treatment, particularly in periodontal care [17]. A recent study investigating the impact of the COVID-19 pandemic on periodontal practice in the UK has shown that one of the main concerns among clinicians was the ability to provide appropriate treatment to patients. According to clinicians, reduced volume of patients per day (76.4%), followed by use of curettes instead of ultrasonic/piezoelectric devices (69.1%) for periodontal treatment, was the top predicted changes during the pandemic [17].
Most of the available studies investigating the use of different devices and/or instruments for subgingival instrumentation focused on NSPT (step 2) [7]. In daily practice, NSPT is always the first choice for treatment of periodontitis patients. However, the prognosis of teeth presenting with periodontitis stages III and IV can be challenging to determine. Although most patients respond positively to NSPT, some areas may still present residual pockets requiring additional surgical treatment (step 3) [3, 5]. Both subgingival instrumentation alone or combined with a flap procedure were considered effective methods for the treatment of residual shallow/moderate pockets (4–5 mm) in terms of PPD reduction and clinical attachment gain. Nevertheless, in the treatment of deep pockets (≥ 6 mm), open flap debridement resulted in greater PPD reduction and clinical attachment gain [3, 25]. Remarkably, Graziani et al. suggested that periodontal surgery not only has a benefit in terms of pocket reduction but also can be a more cost-effective solution for long-term tooth survival than replacement by dental implants [13].
It is important to highlight that the present systematic review included relatively old studies published between 23 and 14 years ago and investigated the use of instruments that may be outdated and do not reflect the instruments/devices currently applied for subgingival instrumentation. There has been a significant evolution especially in terms of ultrasonic tips that are far smaller, thinner, and better performing than the ones used in the past [26]. A recent RCT using a split-mouth design has compared the clinical efficacy, chairside time, and post-treatment hypersensitivity of four instruments used for subgingival periodontal instrumentation during NSPT [19]. Quadrants were randomly divided into four treatments: Gracey curettes (Hu-Friedy®), piezoelectric ultrasonic (Satelec®), diamond burs 40 µm (Intensiv Perioset®), and piezosurgery ultrasonic (Mectron®). At 8 weeks post-intervention, Gracey curettes, piezoelectric ultrasonic (Satelec®), and piezosurgery ultrasonic (Mectron®) were statistically more effective than diamond burs in increasing attachment level and reducing probing pocket depth. Regarding post-treatment hypersensitivity, no statistical differences were observed in any of the groups. Additionally, the ultrasonic scalers showed a significant reduction in chairside time.
Remarkably, there is a lack of studies investigating the efficacy of manual and power-driven instruments when used in combination and how this association could contribute to the clinical endpoints after surgical periodontal treatment. Likewise, no evidence was found comparing different types of instruments for subgingival instrumentation during the surgical phase of periodontal therapy on patient’s comfort or postoperative pain. With an eye towards a more patient-centred approach, it would be important in the future to compare different instruments for root instrumentation and to consider patient-reported outcome measures, including patients’ preferences, discomfort, and perception about therapy. From the present systematic review, it is not possible to conclude if patients are expected to have better or worse PROMs after use of different types of instrumentation during surgical periodontal treatment. However, it is suggested that powered instruments may reduce the length of treatment, and this could be seen as an advantage by both patients and clinicians.
It has been demonstrated that the experience and skill of a clinician can significantly affect the outcome of NSPT performed with the aid of both ultrasonic and hand instruments, with more positive improvement in clinical parameters when performed by a more qualified operator [27]. Hence, the selection of type of instruments may also be influenced by clinicians’ level of skill and experience. While all the studies included in this review were performed in a university setting, under ideal conditions that allowed controlling for some confounding factors, it can be speculated that clinicians that performed the instrumentation were upskilled compared to average general practitioners. Therefore, studies looking at the effectiveness rather than efficacy of root instrumentation with manual vs. powered instruments and performed in general dental practices are warranted.
The present systematic review only allows for assumptions on open flap debridement surgery; however, the impact of different instruments on different types of periodontal surgery is still unknown. Additionally, none of the included studies considered microbiological outcomes and which instrument would present a better performance in terms of decontamination of the root surface. Thus, further studies investigating the use of different instruments combined with novel surgical techniques such as minimally invasive surgical therapy (MIST) or regenerative procedures, where a proper decontamination of the root surface is even more crucial to allow the formation of new attachment, are required. In addition, the impact of different instruments on meaningful endpoints both for the clinician (such as % of pocket closure) and for the patient (like PROMs, adverse events, and tooth survival) should be further investigated.
Conclusion
There are a scarce number of studies assessing the use of different instruments for subgingival instrumentation during periodontal surgery. From the present systematic review, it was concluded that both hand and powered-driven instruments are effective in reducing probing pocket depth after surgical treatment of periodontitis. Hence, the clinician has the option to decide on a case-by-case situation whether to use manual or powered instruments or its combination to achieve a predictable outcome. Aspects to be considered when making this decision should include risk of contamination and development of contaminated aerosols, preferences of the clinician and patient, and time required to perform an adequate decontamination of the root surfaces. The anatomical characteristics of the teeth and easiness of access should also be considered. Further RCTs using new devices are warranted in order to better support the clinical decision-making process, which should consider tangible outcomes both for the clinician (i.e., percentage of closed pockets) and for the patient (i.e., quality of life, risk of adverse events, and tooth survival).
References
Kilian M, Chapple ILC, Hannig M, Marsh PD, Meuric V, Pedersen AML et al (2016) The oral microbiome - an update for oral healthcare professionals. Br Dent J 221(10):657–666. https://doi.org/10.1038/sj.bdj.2016.865
Tonetti MS, Greenwell H, Kornman KS (2018) Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol 45(January):S149–S161. https://doi.org/10.1111/jcpe.12945
Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T et al (2020) Treatment of stage I-III periodontitis—the EFP S3 level clinical practice guideline. J Clin Periodontol 47(S22):4–60. https://doi.org/10.1111/jcpe.13290
Donos N (2000) (2018) The periodontal pocket. Periodontol 76(1):7–15. https://doi.org/10.1111/prd.12203
West N, Chapple I, Claydon N, D’Aiuto F, Donos N, Ide M et al (2021) BSP implementation of European S3 - level evidence-based treatment guidelines for stage I-III periodontitis in UK clinical practice. J Dent 106:103562. https://doi.org/10.1016/j.jdent.2020.103562
Lang NP, Tan WC, Krähenmann MA, Zwahlen M (2008) A systematic review of the effects of full-mouth debridement with and without antiseptics in patients with chronic periodontitis. J Clin Periodontol 35:8–21. https://doi.org/10.1111/j.1600-051X.2008.01257.x
Suvan JE (2000) (2005) Effectiveness of mechanical nonsurgical pocket therapy. Periodontol 37(1):48–71. https://doi.org/10.1111/j.1600-0757.2004.03794.x
Cobb CM (1996) Non-surgical pocket therapy: mechanical. Ann Periodontol 1(1):443–90. https://doi.org/10.1902/annals.1996.1.1.443
Cobb CM (2002) Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J Clin Periodontol 29:22–32. https://doi.org/10.1034/j.1600-051X.29.s2.4.x
Machtei EE, Hausmann E, Schmidt M, Grossi SG, Dunford R, Schifferle R et al (1998) Radiographic and clinical responses to periodontal therapy. J Periodontol 69(5):590–5. https://doi.org/10.1902/jop.1998.69.5.590
Suvan J, Leira Y, Moreno Sancho FM, Graziani F, Derks J, Tomasi C (2020) Subgingival instrumentation for treatment of periodontitis A systematic review. J Clin Periodontol 47(S22):155–175. https://doi.org/10.1111/jcpe.13245
Van der Weijden GA, Timmerman MF (2002) A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol 29:55–71. https://doi.org/10.1034/j.1600-051X.29.s3.3.x
Graziani F, Karapetsa D, Mardas N, Leow N, Donos N (2018) Surgical treatment of the residual periodontal pocket. Periodontol 76(1):150–163. https://doi.org/10.1111/prd.12156
Sallum AW, Alves RV, Teixeira Damis LF, Roesler Bertolini PF, Nociti FH, Sallum EA (2005) Open flap debridement with or without intentional cementum removal: a 4-month follow-up. J Clin Periodontol 32(9):1007–1010. https://doi.org/10.1111/j.1600-051X.2005.00815.x
Johnson IG, Jones RJ, Gallagher JE, Wade WG, Al-Yaseen W, Robertson M et al (2021) Dental periodontal procedures: a systematic review of contamination (splatter, droplets and aerosol) in relation to COVID-19. BDJ Open 7(1):1–7. https://doi.org/10.1038/s41405-021-00070-9
World Health Organization (2020) Transmission of SARS-CoV-2 : implications for infection prevention precautions (July):1–10.
Nibali L, Ide M, Ng D, Buontempo Z, Clayton Y, Asimakopoulou K (2020) The perceived impact of Covid-19 on periodontal practice in the United Kingdom: a questionnaire study. J Dent 102(January):103481. https://doi.org/10.1016/j.jdent.2020.103481
Office of Chief Dental Officer England (2020) Standard operating procedure. Transition to recovery. Available from: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/06/C0575-dental-transition-to-recovery-SOP-4June.pdf. Accessed 21 Mar 2022
Puglisi R, Santos A, Pujol A, Ferrari M, Nart J, Pascual A (2021) Clinical comparison of instrumentation systems for periodontal debridement: a randomized clinical trial. Int J Dent Hyg 20(2):328–338. https://doi.org/10.1111/idh.12520
Higgins JPT, Green S (2011) No Title. In: The Cochrane Collaboration., editor. Cochrane handbook for systematic reviews of interventions [Internet]. Version 5. Available from: http://handbook-5-1.cochrane.org. Accessed 21 Mar 2022
Kocher T, Plagmann HC (1999) Root debridement of single-rooted teeth with a diamond-coated sonic scaler insert during flap surgery: a pilot study. J Clin Periodontol 26(4):201–205. https://doi.org/10.1034/j.1600-051x.1999.260401.x
Kocher T, Plagmann HC (1999) Root debridement of molars with furcation involvement using diamond-coated sonic sealer inserts during flap surgery - a pilot study. J Clin Periodontol 26(8):525–530. https://doi.org/10.1034/j.1600-051x.1999.260806.x
Silva Filho WS, Lima LL, Sallum EA, Sallum AW, Nociti Junior FH, Casati MZ (2008) Avaliação clínica do uso de pontas ultra-sônicas diamantadas na terapia peiodontal não-cirúrgica. Perionews 18:269–274
Ainamo J, Bay I (1975) Problems and proposals for recording gingivitis and plaque. Int Dent J 25(4):229–235
Sanz-sánchez I, Molina A, Aimetti M, Citterio F, Romano F (2020) Efficacy of access flap procedures compared to subgingival debridement in the treatment of periodontitis. A systematic review and meta-analysis. J Clin Periodontol 47:282–302. https://doi.org/10.1111/jcpe.13259
Nibali L, Pometti D, T-t C, Minimally TY, Chen T, Tu Y (2015) Minimally invasive non-surgical approach for the treatment of periodontal intrabony defects : a retrospective analysis. J Clin Periodontol 42:853–9. https://doi.org/10.1111/jcpe.12443
Kozlovsky A, Rapaport A, Artzi Z (2018) Influence of operator skill level on the clinical outcome of non-surgical periodontal treatment: a retrospective study. Clin Oral Investig 22(8):2927–2932. https://doi.org/10.1007/s00784-018-2380-7
Funding
The study was partially supported by the Osteology Foundation, with a research scholarship grant to Jeniffer Perussolo and to the Centre for Oral Clinical Research, Institute of Dentistry, Queen Mary University of London, UK.
Author information
Authors and Affiliations
Contributions
JP, NC, EC, and ND designed the review protocol. JP and NC performed the search and data extraction. MG overviewed data extraction and calculated missing values for primary and secondary outcomes. JP, NC, EC, and ND worked on the data interpretation and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The author Matteo Goldoni has passed away.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Perussolo, J., Cavalli, N., Calciolari, E. et al. Clinical efficacy of hand and power-driven instruments for subgingival instrumentation during periodontal surgical therapy: a systematic review. Clin Oral Invest 27, 1–13 (2023). https://doi.org/10.1007/s00784-022-04759-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04759-5