Abstract
Aim
This study aims to examine the composition of lining and masticatory mucosa at the pre- and post-soft tissue augmentation procedures with a volume-stable cross-linking collagen matrix (VCMX) in humans.
Materials and methods
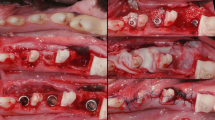
In 12 patients, single implant sites were augmented with a VCMX. Biopsies were obtained including masticatory (MM) and lining (LM) mucosa before augmentation and at 12 weeks post-augmentation procedures. Rete pegs density (RPD), length (RPL), and blood vessel density (BVD) were histomorphometrically analyzed at both time points. Picrosirius red staining under polarized light microscopy was used to evaluate collagen fiber organization. The effects of time and tissue type were evaluated by ANOVA with repeated measures.
Results
Both MM and LM areas demonstrated an increase in mean RPL following augmentation, 382.6 µm ± 95.1 vs. 290.5 µm ± 79.3 and 335.6 µm ± 94.2 vs. 292.9 µm ± 77.0, respectively (p < .05). There was a significant difference in the numbers of RP per 1 mm length (RPD) between the MM (9.2 ± 1.7) and LM (6.1 ± 2.8) mucosa but not between the pre- and post-VCMX augmentation time points. The mean BVD in the LM was greater than in the MM (5.5 ± 2.4 and 6.3 ± 2.4 vs. 3.4 ± 3.3 and 3.7 ± 1.8, respectively, p < .05) but not between time points. The collagen fiber arrangements pre- and post-augmentation were not significantly different.
Conclusion
Augmentation with VCMX did not alter the composition of lining and masticatory mucosa at implant sites.
Clinical relevance
A thick soft tissue phenotype around the implant neck is an important factor to maintain peri-implant health. A non-autogenous cross-linking collagen matrix is proposed as an alternate graft substitute in soft tissue augmentation procedures in order to improve implant soft tissue phenotype.




Similar content being viewed by others
References
Lang NP, Löe H (1972) The relationship between the width of keratinized gingiva and gingival health. J Periodontol 43(10):623–627. https://doi.org/10.1902/jop.1972.43.10.623
Artzi Z, Carmeli G, Kozlovsky A (2006) A distinguishable observation between survival and success rate outcome of hydroxyapatite-coated implants in 5–10 years in function. Clin Oral Implant Res 17(1):85–93. https://doi.org/10.1111/j.1600-0501.2005.01178.x
Chung DM, Oh TJ, Shotwell JL, Misch CE, Wang HL (2006) Significance of keratinized mucosa in maintenance of dental implants with different surfaces. J Periodontol 77(8):1410–1420. https://doi.org/10.1902/jop.2006.050393
Wennström JL, Lindhe J, Sinclair F, Thilander B (1987) Some periodontal tissue reactions to orthodontic tooth movement in monkeys. J Clin Periodontol 14(3):121–129. https://doi.org/10.1111/j.1600-051X.1987.tb00954.x
Hwang D, Wang HL (2006) Flap thickness as a predictor of root coverage: a systematic review. J Periodontol 77(10):1625–1634. https://doi.org/10.1902/jop.2006.060107
Rasperini G, Acunzo R, Cannalire P, Farronato G (2017) Influence of periodontal biotype on root surface exposure during orthodontic treatment: a preliminary study. Int J Periodontics Restorative Dent 35(5):655–675
Zucchelli G, Mounssif I (2015) Periodontal plastic surgery. Periodontol 2000 68(1):333–368. https://doi.org/10.1111/prd.12059
Linkevicius T, Puisys A, Linkeviciene L, Peciuliene V, Schlee M (2015) Crestal bone stability around implants with horizontally matching connection after soft tissue thickening: a prospective clinical trial: thickened soft tissues improve bone stability. Clin Implant Dent Relat Res 17(3):497–508. https://doi.org/10.1111/cid.12155
Suárez-López Del Amo F, Lin GH, Monje A, Galindo-Moreno P, Wang HL (2016) Influence of soft tissue thickness on peri-implant marginal bone loss: a systematic review and meta-analysis. J Periodontol 87(6):690–699. https://doi.org/10.1902/jop.2016.150571
Akcali A, Trullenque-Eriksson A, Sun C, Petrie A, Nibali L, Donos N (2017) What is the effect of soft tissue thickness on crestal bone loss around dental implants? A systematic review. Clin Oral Implants Res 28(9):1046–1053. https://doi.org/10.1111/clr.12916
Jemt T (1997) Regeneration of gingival papillae after single-implant treatment. Int J Periodontics Restorative Dent 17(4):326–333
Buser D, Martin W, Belser UC (2004) Optimizing esthetics for implant restorations in the anterior maxilla: anatomic and surgical considerations. Int J Oral Maxillofac Implants 19(Suppl):43–61
Wiesner G, Esposito M, Worthington H, Schlee M (2010) Connective tissue grafts for thickening peri-implant tissues at implant placement. One-year results from an explanatory split-mouth randomised controlled clinical trial. Eur J Oral Implantol 3(1):27–35
Hämmerle CHF, Araújo MG, Simion M, Osteology Consensus Group 2011 (2012) Evidence-based knowledge on the biology and treatment of extraction sockets. Clin Oral Implants Res. 23(Suppl 5):80–82. https://doi.org/10.1111/j.1600-0501.2011.02370.x
Testori T, Weinstein T, Scutellà F, Wang HL (2000) Zucchelli G (2018) Implant placement in the esthetic area: criteria for positioning single and multiple implants. Periodontol 77(1):176–196. https://doi.org/10.1111/prd.12211
Schwarz F, Giannobile WV, Jung RE (2018) & Groups of the 2nd Osteology Foundation Consensus Meeting. Clin Oral Implant Res 29:11–13
Giannobile WV, Jung RE, Schwarz F, the Groups of the 2nd Osteology Foundation Consensus Meeting (2018) Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: Osteology Foundation Consensus Report Part 1-Effects of soft tissue augmentation procedures on the maintenance of peri-implant soft tissue health. Clin Oral Implants Res 29:7–10
Zigdon H, Machtei EE (2008) The dimensions of keratinized mucosa around implants affect clinical and immunological parameters. Clin Oral Implants Res 19(4):387–392. https://doi.org/10.1111/j.1600-0501.2007.01492.x
Thoma DS, Jung RE, Schneider D et al (2010) Soft tissue volume augmentation by the use of collagen-based matrices: a volumetric analysis: soft tissue volume augmentation. J Clin Periodontol 37(7):659–666. https://doi.org/10.1111/j.1600-051X.2010.01581.x
Schneider D, Grunder U, Ender A, Hämmerle CHF, Jung RE (2011) Volume gain and stability of peri-implant tissue following bone and soft tissue augmentation: 1-year results from a prospective cohort study: volume gain and stability of peri-implant tissue following bone and soft tissue augmentation. Clin Oral Implants Res 22(1):28–37. https://doi.org/10.1111/j.1600-0501.2010.01987.x
Thoma DS, Buranawat B, Hämmerle CHF, Held U, Jung RE (2014) Efficacy of soft tissue augmentation around dental implants and in partially edentulous areas: a systematic review. J Clin Periodontol 41(Suppl 15):S77-91. https://doi.org/10.1111/jcpe.12220
Lin C-Y, Chen Z, Pan WL, Wang HL (2018) Impact of timing on soft tissue augmentation during implant treatment: a systematic review and meta-analysis. Clin Oral Implants Res 29(5):508–521. https://doi.org/10.1111/clr.13148
Yoshino S, Kan JYK, Rungcharassaeng K, Roe P, Lozada JL (2014) Effects of connective tissue grafting on the facial gingival level following single immediate implant placement and provisionalization in the esthetic zone: a 1-year randomized controlled prospective study. Int J Oral Maxillofac Implants 29(2):432–440
Buyukozdemir Askin S, Berker E, Akincibay H et al (2015) Necessity of keratinized tissues for dental implants: a clinical, immunological, and radiographic study: dental implants and keratinized tissue width. Clin Implant Dent Relat Res 17(1):1–12. https://doi.org/10.1111/cid.12079
Fenner N, Hämmerle CHF, Sailer I, Jung RE (2016) Long-term clinical, technical, and esthetic outcomes of all-ceramic vs. titanium abutments on implant supporting single-tooth reconstructions after at least 5 years. Clin Oral Implants Res. 27(6):716–723. https://doi.org/10.1111/clr.12654
Roccuzzo M, Grasso G, Dalmasso P (2016) Keratinized mucosa around implants in partially edentulous posterior mandible: 10-year results of a prospective comparative study. Clin Oral Implant Res 27(4):491–496. https://doi.org/10.1111/clr.12563
Thoma DS, Naenni N, Figuero E et al (2018) Effects of soft tissue augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res 29:32–49. https://doi.org/10.1111/clr.13114
Maksoud MA (2003) Manipulation of the peri-implant tissue for better maintenance: a periodontal perspective. J Oral Implantol 29(3):120–123. https://doi.org/10.1563/1548-1336(2003)029%3c0120:MOTPIT%3e2.3.CO;2
Salvi GE, Lang NP (2004) Diagnostic parameters for monitoring peri-implant conditions. Int J Oral Maxillofac Implants 19(Suppl):116–127
Thoma DS, Benić GI, Zwahlen M, Hämmerle CHF, Jung RE (2009) A systematic review assessing soft tissue augmentation techniques. Clin Oral Implants Res 20(Suppl 4):146–165. https://doi.org/10.1111/j.1600-0501.2009.01784.x
Rojo E, Stroppa G, Sanz-Martin I, Gonzalez-Martín O, Alemany AS, Nart J (2018) Soft tissue volume gain around dental implants using autogenous subepithelial connective tissue grafts harvested from the lateral palate or tuberosity area. A randomized controlled clinical study. J Clin Periodontol 45(4):495–503. https://doi.org/10.1111/jcpe.12869
Farnoush A, A, (1978) Techniques for the protection and coverage of the donor sites in free soft tissue grafts. J Periodontol 49(8):403–5. https://doi.org/10.1902/jop.1978.49.8.403
Griffin TJ, Cheung WS, Zavras AI, Damoulis PD (2006) Postoperative complications following gingival augmentation procedures. J Periodontol 77:2070–2079
Thoma DS, Hämmerle CHF, Cochran DL et al (2011) Soft tissue volume augmentation by the use of collagen-based matrices in the dog mandible – a histological analysis. J Clin Periodontol 38(11):1063–1070
Thoma DS, Villar CC, Cochran DL, Hämmerle CHF, Jung RE (2012) Tissue integration of collagen-based matrices: an experimental study in mice. Clin Oral Implants Res 23(12):1333–1339. https://doi.org/10.1111/j.1600-0501.2011.02356.x
Sanz M, Lorenzo R, Aranda JJ, Martin C, Orsini M (2009) Clinical evaluation of a new collagen matrix (Mucograft prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: a randomized prospective clinical trial. J Clin Periodontol 36(10):868–876. https://doi.org/10.1111/j.1600-051X.2009.01460.x
De Angelis P, De Angelis S, Passarelli PC et al (2020) Clinical comparison of a xenogeneic collagen matrix versus subepithelial autogenous connective tissue graft for augmentation of soft tissue around implants. Int J Oral Maxillofac Surg. https://doi.org/10.1016/j.ijom.2020.11.014
Gargallo-Albiol J, Barootchi S, Tavelli L, Wang HL (2019) Efficacy of xenogeneic collagen matrix to augment peri-implant soft tissue thickness compared to autogenous connective tissue graft: a systematic review and meta-analysis. Int J Oral Maxillofac Implants 34(5):1059–1069
Tavelli L, Barootchi S, Avila-Ortiz G, Urban IA, Giannobile WV, Wang HL (2021) Peri-implant soft tissue phenotype modification and its impact on peri-implant health: a systematic review and network meta-analysis. J Periodontol 92:21–44. https://doi.org/10.1002/JPER.19-0716
Mathes SH, Wohlwend L, Uebersax L et al (2010) A bioreactor test system to mimic the biological and mechanical environment of oral soft tissues and to evaluate substitutes for connective tissue grafts. Biotechnol Bioeng 107(6):1029–1039. https://doi.org/10.1002/bit.22893
Zeltner M, Jung RE, Hämmerle CHF, Hüsler J, Thoma DS (2017) Randomized controlled clinical study comparing a volume-stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: linear volumetric soft tissue changes up to 3 months. J Clin Periodontol 44(4):446–453. https://doi.org/10.1111/jcpe.12697
Thoma DS, Zeltner M, Hilbe M, Hämmerle CHF, Hüsler J, Jung RE (2016) Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J Clin Periodontol 43(10):874–885. https://doi.org/10.1111/clr.13114
Tinti C, Parma-Benfenati S (1995) Coronally positioned palatal sliding flap. Int J Periodontics Restorative Dent 15(3):298–310
Thoma DS, Gasser TJW, Jung RE, Hämmerle CHF (2020) Randomized controlled clinical trial comparing implant sites augmented with a volume-stable collagen matrix or an autogenous connective tissue graft: 3-year data after insertion of reconstructions. J Clin Periodontol 47(5):630–639. https://doi.org/10.1111/jcpe.13271
Song YW, Kim S, Waller T et al (2019) Soft tissue substitutes to increase gingival thickness: histologic and volumetric analyses in dogs. J Clin Periodontol 46(1):96–104. https://doi.org/10.1111/jcpe.13034
Grossman ES, Forbes ME (1990) Studies related to reaction of supporting soft tissue to denture wear: the histological response of vervet monkey oral epithelium to a -80 mmHg vacuum. J Oral Rehabil 17(6):587–597. https://doi.org/10.1111/j.1365-2842.1990.tb01430.x
Wu T, Xiong X, Zhang W, Zou H, Xie H, He S (2013) Morphogenesis of rete ridges in human oral mucosa: a pioneering morphological and immunohistochemical study. Cells Tissues Organs 197(3):239–248. https://doi.org/10.1159/000342926
Junqueira LC, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11(4):447–455
Montes GS, Junqueira LCU (1991) The use of the picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz 86(suppl 3):1–11
Lattouf R, Younes R, Lutomski D et al (2014) Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues: a useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem 62(10):751–758. https://doi.org/10.1369/0022155414545787
Funding
The work was supported partially by Geistlich Pharma AG, Wolhusen, Switzerland.
Author information
Authors and Affiliations
Contributions
Zvi Artzi: concept/design and investigation (lead); project administration (lead); methodology (equal); project administration (lead); data analysis/interpretation, writing—original draft, drafting article, critical revision of article, approval of article, statistics, data collection, and validation (equal); and resources (equal).
Uri Renert: concept/design and investigation (lead); project administration (lead); methodology (equal); project administration (lead); data analysis/interpretation, writing—original draft, drafting article, critical revision of article, approval of article, statistics, data collection, and validation (equal); and resources (equal)
Erez Netanely: concept/design and methodology (equal); data analysis/interpretation, critical revision of article, approval of article, data collection, and validation (equal); and resources (equal)
Daniel S. Thoma: concept/design and investigation (equal); validation (equal); methodology (equal); writing—review and editing (equal); and resources (equal)
Marilena Vered: concept/design and methodology (equal); data analysis/interpretation critical revision of article, approval of article, data collection, and supervision (equal); validation (equal); and resources (equal)
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.. This study was approved by the Tel Aviv University ethics committee (approval no. 134.18). Enclosed separately the original confirmation dated January 08, 2019.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Artzi, Z., Renert, U., Netanely, E. et al. Histologic composition of marginal mucosal tissue augmented by a resorbable volume-stable collagen matrix in soft tissue thickening procedures in humans: a morphometric observational study. Clin Oral Invest 26, 427–435 (2022). https://doi.org/10.1007/s00784-021-04016-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04016-1