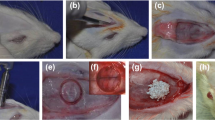
Abstract
Objectives
The aim of this study was to investigate if bone regeneration can be promoted by homologous transplantation of STRO-1 sorted (STRO-1+) porcine tooth germ mesenchymal stem cells (TGSCs) with the combination of polyethylenglycol (PEG)-based hydrogel and biphasic calcium phosphate (BCP) scaffolds.
Material and methods
TGSCs were isolated from impacted third molars of domestic pigs. Nine critical-sized defects were created as (1) untreated defect; filled with (2) autogenous bone; (3) BCP + PEG; (4) BCP + PEG + unsorted TGSCs; (5) BCP + unsorted TGSCs; (6) BCP + PEG + STRO-1-sorted TGSCs; (7) BCP + STRO-1-sorted TGSCs; (8) BCP + PEG + osteogenic induced unsorted TGSCs; and (9) BCP + PEG + osteogenic induced STRO-1-sorted TGSCs in 20 domestic pigs. CM-DiI labelling was used to track cells in vivo. Histomorphometric assessment of new bone formation was achieved by toluidine blue O staining and microradiography after 1, 2, 4 and 12 weeks posttransplantation.
Results
Complete healing was achieved in all defects although defects with PEG hydrogel presented better bone formation while STRO-1+ and unsorted TGSCs showed similar ability to form new bone after 12 weeks. Transplanted cells were seen in defects where PEG hydrogel was used as carriers in contrast to defects treated with cells and only bone grafts.
Conclusions
PEG hydrogel is an efficient carrier for homologous stem cell transplantation. TGSCs are capable of promoting bone healing in critical-sized defects in combination with bone graft and PEG hydrogel.
Clinical relevance
This study provides information about the importance of the delivery vehicle for future translational stem cell delivery approaches.










Similar content being viewed by others
References
Cordeiro PG, Disa JJ, Hidalgo DA, Hu QY (1999) Reconstruction of the mandible with osseous free flaps: a 10-year experience with 150 consecutive patients. Plast Reconstr Surg 104:1314–1320. https://doi.org/10.1097/00006534-199910000-00011
Chang YM, Chana JS, Wei FC, Tsai CY, Chen SH (2003) Osteotomy to treat malocclusion following reconstruction of the mandible with the free fibula flap. Plast Reconstr Surg 112:31–36. https://doi.org/10.1097/01.PRS.0000065910.66161.DD
von Wilmowsky C, Schwarz S, Kerl JM, Srour S, Lell M, Felszeghy E, Schlegel KA (2010) Reconstruction of a mandibular defect with autogenous, autoclaved bone grafts and tissue engineering: an in vivo pilot study. J Biomed Mater Res A 93:1510–1518. https://doi.org/10.1002/jbm.a.32635
Schlegel KA, Lang FJ, Donath K, Kulow JT, Wiltfang J (2006) The monocortical critical size bone defect as an alternative experimental model in testing bone substitute materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:7–13. https://doi.org/10.1016/j.tripleo.2005.09.011
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. J Biomat Sci-Polym E 32(8-9):762–798. https://doi.org/10.1016/j.progpolymsci.2007.05.017
Niemeyer P, Szalay K, Luginbühl R, Südkamp NP, Kasten P (2010) Transplantation of human mesenchymal stem cells in a non-autogenous setting for bone regeneration in a rabbit critical-size defect model. Acta Biomater 6:900–908. https://doi.org/10.1016/j.actbio.2009.09.007
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49. https://doi.org/10.1038/nature00870
Ben-Ari A, Rivkin R, Frishman M, Gaberman E, Levdansky L, Gorodetsky R (2009) Isolation and implantation of bone marrow-derived mesenchymal stem cells with fibrin micro beads to repair a critical-size bone defect in mice. Tissue Eng Part A 15:2537–2546. https://doi.org/10.1089/ten.tea.2008.0567
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630. https://doi.org/10.1073/pnas.240309797
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S, SHED (2003) stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100:5807–5812. https://doi.org/10.1073/pnas.0937635100
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155. https://doi.org/10.1016/S0140-6736(04)16627-0
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166–171. https://doi.org/10.1016/j.joen.2007.11.021
Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, Sippel C, Hoffmann KH (2005) Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 24:155–165. https://doi.org/10.1016/j.matbio.2004.12.004
Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, Tadokoro M, Katsube Y, Isoda K, Kondoh M, Kawase M, Go MJ, Adachi H, Yokota Y, Kirita T, Ohgushi H (2008) Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation 76(5):495–505. https://doi.org/10.1111/j.1432-0436.2007.00245.x
Yalvac ME, Ramazanoglu M, Rizvanov AA, Sahin F, Bayrak OF, Salli U, Palotás A, Kose GT (2010) Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: implications in neo-vascularization, osteo-, adipo- and neurogenesis. Pharm J 10:105–113. https://doi.org/10.1038/tpj.2009.40
Ercal P, Pekozer GG, Gumru OZ, Kose GT, Ramazanoglu M (2017) Influence of STRO-1 selection on osteogenic potential of human tooth germ derived mesenchymal stem cells. Arch Oral Biol 82:293–301. https://doi.org/10.1016/j.archoralbio.2017.06.028
Gurel Pekozer G, Ramazanoglu M, Schlegel KA, Kok FN, Torun Kose G (2018) Role of STRO-1 sorting of porcine dental germ stem cells in dental stem cell-mediated bone tissue engineering. Artif Cells Nanomed Biotechnol 46(3):607–618. https://doi.org/10.1080/21691401.2017.1332637
Kawate K, Yajima H, Ohgushi H, Kotobuki N, Sugimoto K, Ohmura T, Kobata Y, Shigematsu K, Kawamura K, Tamai K, Takakura Y (2006) Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs 30:960–962. https://doi.org/10.1111/j.1525-1594.2006.00333.x
Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, Le AD, Wang SL (2009) Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res 88:249–254. https://doi.org/10.1177/0022034509333804
Frame JW (1987) Hydroxyapatite as a biomaterial for alveolar ridge augmentation. Int J Oral Maxillofac Surg 16:642–655. https://doi.org/10.1016/s0901-5027(87)80048-6
Misch CE, Dietsh F (1993) Bone grafting materials in implant dentistry. Implant Dent 2:158–167. https://doi.org/10.1097/00008505-199309000-00003
Fetner AE, Hartigan MS, Low SB (1994) Periodontal repair using PerioGlas in nonhuman primates: clinical and histologic observations. Compendium 15(932):935–938 quiz 939
Wehrhan F, Amann K, Molenberg A, Lutz R, Neukam FW, Schlegel KA (2013) Critical size defect regeneration using PEG-mediated BMP-2 gene delivery and the use of cell occlusive barrier membranes - the osteopromotive principle revisited. Clin Oral Implants Res 24(8):910–920. https://doi.org/10.1111/j.1600-0501.2012.02489.x
Donath K, Breuner G (1982) A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol 11:318–326. https://doi.org/10.1111/j.1600-0714.1982.tb00172.x
Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88(9):792–806. https://doi.org/10.1177/0022034509340867
Cobourne MT, Sharpe PT (2003) Tooth and jaw: molecular mechanisms of patterning in the first branchial arch. Arch Oral Biol 48(1):1–14. https://doi.org/10.1016/s0003-9969(02)00208-x
Yildirim S (2013) Tooth development. In: Dental Pulp Stem Cells, New York, NY:Springer, pp. 5–16. https://doi.org/10.1007/978-1-4614-5687-2
Wang F, Xiao J, Cong W, Li A, Song T, Wei F, Xu J, Zhang C, Fan Z, Wang S (2014) Morphology and chronology of diphyodont dentition in miniature pigs, Sus Scrofa. Oral Dis 20(4):367–379. https://doi.org/10.1111/odi.12126
Wang S, Liu Y, Fang D, Shi S (2007) The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis 13(6):530–537. https://doi.org/10.1111/j.1601-0825.2006.01337.x
Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1:e79. https://doi.org/10.1371/journal.pone.0000079
Lindroos B, Maenpaa K, Ylikomi T, Oja H, Suuronen R, Miettinen S (2008) Characterization of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun 368:329–335. https://doi.org/10.1016/j.bbrc.2008.01.081
Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, An Y, Zhang C, Wang S (2010) Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells 28:1829–1838. https://doi.org/10.1002/stem.512
Gothard D, Greenhough J, Ralph E, Oreffo RO (2014) Prospective isolation of human bone marrow stromal cell subsets: a comparative study between Stro-1-, CD146- and CD105-enriched populations. J Tissue Eng 5:2041731414551763. https://doi.org/10.1177/2041731414551763
Lv FJ, Tuan RS, Cheung KM, Leung VY (2014) Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32(6):1408–1419. https://doi.org/10.1002/stem.1681
Salinas CN, Anseth KS (2009) Mesenchymal stem cells for craniofacial tissue regeneration: designing hydrogel delivery vehicles. J Dent Res 88(8):681–692. https://doi.org/10.1177/0022034509341553
Wehrhan F, Amann K, Molenberg A, Lutz R, Neukam FW, Schlegel KA (2012) PEG matrix enables cell-mediated local BMP-2 gene delivery and increased bone formation in a porcine critical size defect model of craniofacial bone regeneration. Clin Oral Implants Res 23(7):805–813. https://doi.org/10.1111/j.1600-0501.2011.02223.x
Jones TD, Kefi A, Sun S, Cho M, Alapati SB (2016) An optimized injectable hydrogel scaffold supports human dental pulp stem cell viability and spreading. Adv Med 2016:7363579–7363578. https://doi.org/10.1155/2016/7363579
Jung RE, Weber FE, Thoma DS, Ehrbar M, Cochran DL, Hämmerle CH (2008) Bone morphogenetic protein-2 enhances bone formation when delivered by a synthetic matrix containing hydroxyapatite/tricalciumphosphate. Clin Oral Implants Res 19(2):188–195. https://doi.org/10.1111/j.1600-0501.2007.01431.x
Catros S, Molenberg A, Freilich M, Dard M (2015) Evaluation of a polyethylene glycol-osteogenic protein-1 system on alveolar bone regeneration in the mini-pig. J Oral Implantol 41(4):e96–e10. https://doi.org/10.1563/aaid-joi-D-13-00307
Thoma DS, Schneider D, Mir-Mari J, Hammerle CH, Gemperli AC, Molenberg A, Dard M, Jung RE (2014) Biodegradation and bone formation of various polyethylene glycol hydrogels in acute and chronic sites in mini-pigs. Clin Oral Implants Res 25(4):511–521. https://doi.org/10.1111/clr.12203
Garcia JR, Clark AY, Garcia AJ (2016) Integrin-specific hydrogels functionalized with VEGF for vascularization and bone regeneration of critical-size bone defects. J Biomed Mater Res A 104(4):889–900. https://doi.org/10.1002/jbm.a.35626
Thoma DS, Weber FE, Bienz SP, Hammerle CHF, Jung RE (2017) Biodegradation and tissue integration of various polyethylene glycol matrices: a comparative study in rabbits. Clin Oral Implants Res 28(11):e244–e251. https://doi.org/10.1111/clr.13004
Carles-Carner M, Saleh LS, Bryant SJ (2018) The effects of hydroxyapatite nanoparticles embedded in a MMP-sensitive photoclickable PEG hydrogel on encapsulated MC3T3-E1 pre-osteoblasts. Biomed Mater 13(4):045009
Kar M, Vernon Shih YR, Velez DO, Cabrales P, Varghese S (2016) Poly(ethylene glycol) hydrogels with cell cleavable groups for autonomous cell delivery. Biomaterials 77:186–197. https://doi.org/10.1016/j.biomaterials.2015.11.018
Smith EL, Kanczler JM, Gothard D, Roberts CA, Wells JA, White LJ, Qutachi O, Sawkins MJ, Peto H, Rashidi H, Rojo L, Stevens MM, El Haj AJ, Rose FR, Shakesheff KM, Oreffo RO (2014) Evaluation of skeletal tissue repair, part 2: enhancement of skeletal tissue repair through dual-growth-factor-releasing hydrogels within an ex vivo chick femur defect model. Acta Biomater 10(10):4197–4205. https://doi.org/10.1016/j.actbio.2014.05.025
Castano-Izquierdo H, Alvarez-Barreto J, van den Dolder J, Jansen JA, Mikos AG, Sikavitsas VI (2007) Pre-culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res A 82(1):129–138. https://doi.org/10.1002/jbm.a.31082
Gruber RM, Krohn S, Mauth C, Dard M, Molenberg A, Lange K, Preske C, Schliephake H (2014) Mandibular reconstruction using a calcium phosphate/polyethylene glycol hydrogel carrier with BMP-2. J Clin Periodontol 41(8):820–826. https://doi.org/10.1111/jcpe.12264
Shin H, Quinten Ruhe P, Mikos AG, Jansen JA (2003) In vivo bone and soft tissue response to injectable, biodegradable oligo(poly(ethylene glycol) fumarate) hydrogels. Biomaterials 24(19):3201–3211. https://doi.org/10.1016/s0142-9612(03)00168-6
Asawa Y, Sakamoto T, Komura M, Watanabe M, Nishizawa S, Takazawa Y, Takato T, Hoshi K (2012) Early stage foreign body reaction against biodegradable polymer scaffolds affects tissue regeneration during the autologous transplantation of tissue-engineered cartilage in the canine model. Cell Transplant 21:1431–1442. https://doi.org/10.3727/096368912X640574
Saleh LS, Carles-Carner M, Bryant SJ (2018) The in vitro effects of macrophages on the osteogenic capabilities of MC3T3-E1 cellsencapsulated in a biomimetic poly(ethylene glycol) hydrogel. Acta Biomater 71:37–48. https://doi.org/10.1016/j.actbio.2018.02.026
van der Stok J, Koolen MK, Jahr H, Kops N, Waarsing JH, Weinans H, van der Jagt OP (2014) Chondrogenically differentiated mesenchymal stromal cell pellets stimulate endochondral bone regeneration in critical-sized bone defects. Eur Cell Mater 27:137–148. https://doi.org/10.22203/ecm.v027a11
Acknowledgments
We would like to thank Aart Molenberg (Straumann Company) for supplying us with the PEG hydrogels and the ITI International Team for Implantology (750-2011) for granting our work. The animal studies were performed in cooperation with the Semmelweis-University, Budapest, Hungary. Specimen processing was undertaken in the laboratories of the Department of Oral and Maxillofacial Surgery, University of Erlangen-Nürnberg, Erlangen, Germany. The authors have no conflicts of interest. The work of Dr. Endre Felszeghy, Elke Diebel, and Andrea Schönherr is highly appreciated.
Funding
The work was supported by the ITI International Team for Implantology (750-2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study was approved by Agricultural Administrative Office of the Capital and Country Pest, Food Chain Security Management, Department of Epidemic and Animal Welfare (approval no. 22.1/3879/003/2008). Animal care keeping and surgical procedures were performed in the European Animal Research Centre (“EARC”; 2053 Herceghalom, Hungary, Gesztenyes ut 1; Certified for “Biological evaluation of medical devices” (EN ISO 10993-2:2006).
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure
Osteogenic, adipogenic and chondrogenic differentiation of pTGSCs; Microscopic observation of Alizarin Red (Scale bar 200 μm), Alcian Blue (Scale bar 50 μm), Oil Red O (Scale bar 50 μm) and von Kossa (Scale bar 100 μm) stained pTGSCs under bright field microscope after 21 days in differentiation medium. (PNG 27995 kb)
Rights and permissions
About this article
Cite this article
Ramazanoglu, M., Moest, T., Ercal, P. et al. The effect of polyethylenglycol gel on the delivery and osteogenic differentiation of homologous tooth germ–derived stem cells in a porcine model. Clin Oral Invest 25, 3043–3057 (2021). https://doi.org/10.1007/s00784-020-03625-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03625-6