Abstract
Objectives
The objective of this study was to evaluate the changes that can occur in saliva components in patients with Alzheimer’s disease (AD) of different severity and determine if any of these components could be a biomarker of this disease. Therefore, a panel of selected analytes related to the amyloid cascade, the immune and adrenergic systems, among others, were analyzed in the saliva of patients with Alzheimer’s disease.
Methods
A total of 152 patients with AD and controls were included. The severity of the disease was established according to the Global Deterioration Scale. Unstimulated whole saliva was collected.
Results
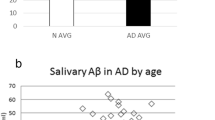
Salivary amyloid-β42 was significantly lower, and complement C4 was significantly higher in the patients with AD than in the controls (p < 0.05 in both cases). Only complement C4 maintained its significant effect in the multivariate regression analysis. However, the area under the receiver operating characteristic curve of C4 was 0.613. No changes were found in any analyte regarding the severity of the disease.
Conclusions
A decrease in amyloid-β42 and an increase in complement C4 were detected in the saliva of patients with AD, but the changes did not show a high diagnostic performance for the detection of AD and were not associated with its severity.
Clinical relevance
Although some analytes showed significant differences in saliva in patients with AD, in our study conditions the salivary biomarkers analyzed were not of enough diagnostic utility for being used in routine.


Similar content being viewed by others
References
Cummings JL (2004) Alzheimer’s disease. N Engl J Med 351:56–67. https://doi.org/10.1056/NEJMra040223
Dubois B, Albert ML (2004) Amnestic MCI or prodromal Alzheimer’s disease? Lancet Neurol 3:246–248. https://doi.org/10.1016/S1474-4422(04)00710-0
Blennow K, Hampel H (2003) CSF markers for incipient Alzheimer’s disease. Lancet Neurol 2:605–613. https://doi.org/10.1016/S1474-4422(03)00530-1
Dugger BN, Whiteside CM, Maarouf CL, Walker DG, Beach TG, Sue LI, Garcia A, Dunckley T, Meechoovet B, Reiman EM, Roher AE (2016) The presence of select tau species in human peripheral tissues and their relation to Alzheimer’s disease. J Alzheimers Dis 51:345–356. https://doi.org/10.3233/JAD-150859
Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, Hölttä M, Rosén C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, Zetterberg H (2016) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 15:673–684. https://doi.org/10.1016/S1474-4422(16)00070-3
Prvulovic D, Hampel H (2011) Amyloid β (Aβ) and phospho-tau (p-tau) as diagnostic biomarkers in Alzheimer’s disease. Clin Chem Lab Med 49:367–374. https://doi.org/10.1515/CCLM.2011.087
Snider BJ, Fagan AM, Roe C, Shah AR, Grant EA, Xiong C, Morris JC, Holtzman DM (2009) Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol 66:638–645. https://doi.org/10.1001/archneurol.2009.55
Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C (2011) Diagnostic potential of saliva: current state and future applications. Clin Chem 57:675–687. https://doi.org/10.1373/clinchem.2010.153767
Lee M, Guo J-P, Kennedy K et al (2016) A method for diagnosing Alzheimer’s disease based on salivary amyloid-β protein 42 levels. J Alzheimers Dis 55:1175–1182. https://doi.org/10.3233/JAD-160748
Bermejo-Pareja F, Antequera D, Vargas T, Molina JA, Carro E (2010) Saliva levels of Abeta1-42 as potential biomarker of Alzheimer’s disease: a pilot study. BMC Neurol 10:108. https://doi.org/10.1186/1471-2377-10-108
Hilal S, Ikram MA, Verbeek MM, Franco OH, Stoops E, Vanderstichele H, Niessen WJ, Vernooij MW (2018) C-reactive protein, plasma amyloid-β levels, and their interaction with magnetic resonance imaging markers. Stroke 49:2692–2698. https://doi.org/10.1161/STROKEAHA.118.022317
Dong MX, Xu XM, Hu L et al (2017) Serum butyrylcholinesterase activity: a biomarker for Parkinson’s disease and related dementia. Biomed Res Int 2017. https://doi.org/10.1155/2017/1524107
Sabbagh MN, Shi J, Lee M, Arnold L, al-Hasan Y, Heim J, McGeer P (2018) Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: preliminary findings. BMC Neurol 18:155. https://doi.org/10.1186/s12883-018-1160-y
Shi M, Sui Y-T, Peskind ER, Li G, Hwang H, Devic I, Ginghina C, Edgar JS, Pan C, Goodlett DR, Furay AR, Gonzalez-Cuyar LF, Zhang J (2011) Salivary tau species are potential biomarkers of Alzheimer’s disease. J Alzheimers Dis 27:299–305. https://doi.org/10.3233/JAD-2011-110731
Pekeles H, Qureshi HY, Paudel HK, Schipper HM, Gornistky M, Chertkow H (2019) Development and validation of a salivary tau biomarker in Alzheimer’s disease. Alzheim Dement Diagn Assess Dis Monit 11:53–60. https://doi.org/10.1016/J.DADM.2018.03.003
Rosén C, Hansson O, Blennow K, Zetterberg H (2013) Fluid biomarkers in Alzheimer’s disease–current concepts. Mol Neurodegener 8:20. https://doi.org/10.1186/1750-1326-8-20
Wormwood KL, Aslebagh R, Channaveerappa D, Dupree EJ, Borland MM, Ryan JP, Darie CC, Woods AG (2015) Salivary proteomics and biomarkers in neurology and psychiatry. PROTEOMICS - Clin Appl 9:899–906. https://doi.org/10.1002/prca.201400153
Sardi F, Fassina L, Venturini L, Inguscio M, Guerriero F, Rolfo E, Ricevuti G (2011) Alzheimer’s disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun Rev 11:149–153. https://doi.org/10.1016/J.AUTREV.2011.09.005
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57:1105–1121. https://doi.org/10.3233/JAD-161088
Anand P, Singh B (2013) A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharm Res 36:375–399. https://doi.org/10.1007/s12272-013-0036-3
Schraen-Maschke S, Sergeant N, Dhaenens C-M, Bombois S, Deramecourt V, Caillet-Boudin ML, Pasquier F, Maurage CA, Sablonnière B, Vanmechelen E, Buée L (2008) Tau as a biomarker of neurodegenerative diseases. Biomark Med 2:363–384. https://doi.org/10.2217/17520363.2.4.363
Reisberg B (1988) Global Deterioration Scale (GDS). Psychopharmacol Bull 24:661–663
Tvarijonaviciute A, Castillo C, Ceron JJ et al (2017) Leptin and NGF in saliva of patients with diabetes mellitus type 2: a pilot study. J Oral Pathol Med 46. https://doi.org/10.1111/jop.12587
Lopez-Jornet P, Cayuela CA, Tvarijonaviciute A et al (2016) Oral lichen planus: Salival biomarkers cortisol, immunoglobulin A, adiponectin. J Oral Pathol Med 45. https://doi.org/10.1111/jop.12345
Delacour H, Bousquet A, Fontan E, Ceppa F (2013) Ammonia does not interfere with the diazyme adenosine deaminase test. Clin Chem Lab Med 51:e225–e226. https://doi.org/10.1515/cclm-2013-0115
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/ABIO.1996.0292
Tecles F, Escribano D, Martínez-Miró S, Hernández F, Contreras MD, Cerón JJ (2016) Cholinesterase in porcine saliva: analytical characterization and behavior after experimental stress. Res Vet Sci 106:23–28. https://doi.org/10.1016/J.RVSC.2016.03.006
Buerger K, Alafuzoff I, Ewers M, Pirttilä T, Zinkowski R, Hampel H (2007) No correlation between CSF tau protein phosphorylated at threonine 181 with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 130:e82–e82. https://doi.org/10.1093/brain/awm140
Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L (2006) Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5:228–234. https://doi.org/10.1016/S1474-4422(06)70355-6
Buerger K, Ewers M, Pirttila T et al (2006) CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 129:3035–3041. https://doi.org/10.1093/brain/awl269
Barranco T, Cerón JJ, López-Jornet P et al (2018) Impact of saliva collection and processing methods on aspartate aminotransferase, creatin kinase and lactate dehydrogenase activities. Anal Sci 34. https://doi.org/10.2116/analsci.17N035
Blennow K, Hampel H, Weiner M, Zetterberg H (2010) Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 6:131–144. https://doi.org/10.1038/nrneurol.2010.4
Daborg J, Andreasson U, Pekna M, Lautner R, Hanse E, Minthon L, Blennow K, Hansson O, Zetterberg H (2012) Cerebrospinal fluid levels of complement proteins C3, C4 and CR1 in Alzheimer’s disease. J Neural Transm 119:789–797. https://doi.org/10.1007/s00702-012-0797-8
Oh YS, Turner RJ (2006) Effect of γ-secretase inhibitors on muscarinic receptor-mediated calcium signaling in human salivary epithelial cells. Am J Physiol Physiol 291:C76–C82. https://doi.org/10.1152/ajpcell.00508.2005
Wang J, Schipper HM, Velly AM, Mohit S, Gornitsky M (2015) Salivary biomarkers of oxidative stress: a critical review. Free Radic Biol Med 85:95–104. https://doi.org/10.1016/J.FREERADBIOMED.2015.04.005
Nerl C, Mayeux R, O’Neill GJ (1984) HLA-linked complement markers in Alzheimer’s and Parkinson’s disease: C4 variant (C4B2) a possible marker for senile dementia of the Alzheimer type. Neurology 34:310–314. https://doi.org/10.1212/wnl.34.3.310
Eikelenboom P, Hack CE, Rozemuller JM, Stam FC (1989) Complement activation in amyloid plaques in Alzheimer’s dementia. Virchows Arch B Cell Pathol Incl Mol Pathol 56:259–262
Bergamaschini L, Donarini C, Gobbo G, Parnetti L, Gallai V (2001) Activation of complement and contact system in Alzheimer’s disease. Mech Ageing Dev 122:1971–1983. https://doi.org/10.1016/S0047-6374(01)00311-6
Eikelenboom P, Veerhuis R (1996) The role of complement and activated microglia in the pathogenesis of Alzheimer’s disease. Neurobiol Aging 17:673–680
Klaver AC, Coffey MP, Bennett DA, Loeffler DA (2017) Specific serum antibody binding to phosphorylated and non-phosphorylated tau in non-cognitively impaired, mildly cognitively impaired, and Alzheimer’s disease subjects: an exploratory study. Transl Neurodegener 6:32. https://doi.org/10.1186/s40035-017-0100-x
Ghag G, Bhatt N, Cantu DV, Guerrero-Munoz MJ, Ellsworth A, Sengupta U, Kayed R (2018) Soluble tau aggregates, not large fibrils, are the toxic species that display seeding and cross-seeding behavior. Protein Sci 27:1901–1909. https://doi.org/10.1002/pro.3499
Acknowledgments
The authors would like to acknowledge all participants of the study. Ministry of Economy and Competitiveness of Spain supported AT through a postdoctoral program “Ramon y Cajal” (RYC-2017-22992). This research was funded by the Seneca foundation of Murcia Regional Government (Spain), grantnumber 19894/GERM/15.
Author information
Authors and Affiliations
Contributions
Conceptualization, PLJ, JJC, and AT; methodology, PLJ, AT, and CZ; formal analysis, CZ, AT, SMS, and LPM; investigation, PLJ, CZ, AT, SMS, LPM, and JJC; resources, PLJ and JJC; data curation, AT and AFBC; writing–original draft preparation, CZ, PLJ, AT; writing–review and editing, PLJ, AT, JJC, SMS; supervision, PLJ and AT; funding acquisition, PLJ and JJC.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study and/or their relatives or legal representatives.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tvarijonaviciute, A., Zamora, C., Ceron, J.J. et al. Salivary biomarkers in Alzheimer’s disease. Clin Oral Invest 24, 3437–3444 (2020). https://doi.org/10.1007/s00784-020-03214-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03214-7