Abstract
Objectives
The ultrasonic NO PAIN technology (Electro Medical Systems, Nyon, CH) promises minimal pain during debridement due to linear oscillating action combined with a sinusoidal power output and feedback control. The aim of the present study was to measure pain perception on a visual analogue scale (VAS) during supportive periodontal therapy including debridement of hypersensitive teeth. Two ultrasonic scalers were used, one with and one without NO PAIN technology.
Material and methods
In a randomized-controlled clinical study with split-mouth design, 100 hypersensitive teeth matched for air blast hypersensitivity were either treated with the ultrasonic device Piezon Master 700 or the Mini Piezon (both EMS, Nyon, CH). Pain perception during debridement was assessed by a VAS (range 0–10).
Results
The average VAS for the test device Piezon Master 700 with NO PAIN technology was 3.16 ± 2.10, and for the control device Mini Piezon without NO PAIN technology 3.40 ± 2.59 (p = 0.490). Placing an arbitrary threshold at the VAS score of 3 for significant pain experience, 60 % of the subjects experienced no significant pain with either instrument.
Conclusion
No statistically significant difference in perceived pain between the instruments used was found.
Clinical relevance
Both ultrasonic devices showed very small pain intensities during debridement of highly hypersensitive teeth and can therefore be recommended for supportive periodontal therapy.
Similar content being viewed by others
Background
The Consensus report of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases reinforced the need to enrol patients treated for periodontitis in a supportive periodontal therapy regimen [1]. Supportive periodontal therapy aims at preventing the recurrence of periodontal disease in terms of tooth loss and additional attachment loss through periodic preventive interventions [2, 3]. Such regimen includes routine assessments of disease and oral hygiene status, behaviour modification and professional mechanical plaque and calculus removal (PMPR) [2]. The importance and effectiveness of supportive periodontal therapy in the secondary prevention of periodontal disease have been well established [1]. The authors of the Consensus report of the 11th European Workshop on Periodontology concluded that patients treated for periodontitis can maintain their dentition with limited variations in periodontal parameters when regularly complying with a supportive periodontal therapy regimen based on routine PMPR [1]. Additionally, patients irregularly complying with the planned supportive periodontal therapy regimen showed greater tooth loss and disease progression when compared to patients who comply regularly [1, 4].
Pain during PMPR was recently reported to be a significant factor influencing clinical compliance to periodontal therapy [5]. The healing of periodontal tissues after active periodontal therapy often results in gingival recession, and in addition, root debridement leads to loss of cementum [6, 7]. The short, sharp pain arising from exposed dentin in response to thermal, tactile, osmotic or chemical stimuli has been defined as dentin hypersensitivity [8]. Levels of dentin hypersensitivity may increase after surgical as well as non-surgical periodontal treatment [9–11]. Among periodontal patients, the occurrence of root sensitivity has been reported to reach up to 98 % [12]. PMPR often induces dentin hypersensitivity due to thermal or tactile stimuli. The ability to deliver dental care with a minimum of patient discomfort should be an essential part of a clinician’s skills to avoid a decline of compliance with supportive periodontal therapy [13].
The EMS Piezon® NO PAIN technology (Electro Medical Systems (EMS), Nyon, CH) promises minimal pain during PMPR and no injury of the gingiva, due to controlled linear oscillating instrument movements parallel to the tooth surface, combined with a sinusoidal power output and feedback control [14].
The aim of the present randomized-controlled clinical study was to compare subjective pain intensities during PMPR of hypersensitive teeth with two piezoelectric ultrasonic devices, one including NO PAIN technology (Piezon Master 700, EMS, Nyon, CH), and one without (Mini Piezon, EMS, Nyon, CH).
Material and methods
Ethical considerations
The Ethics Committee of the Medical University of Innsbruck, Austria, approved the study. The study was conducted in accordance with the 1964 Helsinki declaration and its later amendments. All subjects signed an informed written consent prior to the study enrolment.
Study subjects
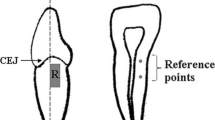
For the study, 53 patients of the dental clinic of the Medical University of Innsbruck who were known for generalized and severe dentin hypersensitivity were recruited (Fig. 1). Subjects had to exhibit a minimum of two hypersensitive teeth in two different quadrants. For the respective teeth, an air blast stimuli score of 2 or 3 (Schiff Cold Air Sensitivity Scale - SCASS) had to be present at the baseline examination. Both teeth of the subject had to feature the same air blast stimuli score. Additionally, test and control teeth had to be from the same tooth category (category 1: incisive, category 2: canines and premolars, category 3: molars). Any teeth with cracked enamel, enamel defects, caries, or extensive/defective restorations, clinically diagnosed pulpitis, and teeth with orthodontic appliances were excluded. Additional exclusion criteria encompassed subjects with gross oral pathology, PMPR or orthodontic treatment within the last 3 months, subjects with eating disorders, as well as pregnant or lactating women, and psychiatric disorders. Current users of anticonvulsants, antihistamines, antidepressants, sedatives, tranquillisers, anti-inflammatory drugs or daily analgesics were also excluded.
Clinical intervention
At baseline, one investigator (HH) measured tactile and air blast hypersensitivity and selected two hypersensitive teeth in two different quadrants with SCASS 2 or 3. For SCASS, the test and control teeth were isolated from the adjacent teeth by the placement of red boxing wax. Air was delivered from a standard dental unit air syringe at maximal pressure (45 psi) and at an environmental temperature of 19–24°. Air was applied for 1 s at a distance of 1 cm perpendicular to the buccal surface of the tooth. The SCASS was used to assess the subject’s response to the stimulus. The scale is graduated into four units: 0 = subject does not respond to the stimulus; 1 = subject does not respond to the stimulus, but considers stimulus to be painful; 2 = subject responds to air stimulus, but does not move away from the stimulus; 3 = subject responds significantly to air stimulus, moves away from the stimulus, and requests immediate termination of the stimulus [15]. Patients were informed before testing about the different units of the scale. Tactile hypersensitivity was assessed by scratching on the dentinal surface with a sharp-tipped probe and a maximum pressure of 70 g. Pressure of 70 g was calibrated with a letter balance before each investigation. The subjects graded pain intensity on a visual analogue scale (VAS) (0 = no pain and 10 = extreme, unbearable pain). Patients were instructed to point at the VAS. Probing pocket depths recorded at six sites per tooth were available from all patients. Recession depth was measured on the buccal aspect of the tooth.
Both teeth were matched for air blast hypersensitivity and were randomly assigned by the second investigator (MS) to one of two treatment groups by rolling a dice: (1) supragingival debridement for 30 s using an ultrasonic scaler with the specific NO PAIN technology (Piezon Master 700, EMS, Nyon, CH), or (2) supragingival debridement for 30 s using an ultrasonic scaler without the NO PAIN technology (Mini Piezon, EMS, Nyon, CH). For both devices, the same tip was used (EMS Instrument PS). According to oral advice of the manufacturer, the power of both devices was set to 50 %. Temperature of physiological saline solution and water for cooling was adjusted to 24 °C. During debridement, the lower end of the tip was applied from coronal to apical with minimal pressure using brushing strokes parallel to the tooth surface. The blinded investigator HH performed the follow-up examinations: Intervention blinded patients were asked to protocol their perception of the instrumentation immediately after the treatment (main outcome measure) on an interval scale (VAS) ranging from 0, representing no pain or discomfort, to 10, representing maximum pain and discomfort.
Statistical analyses
Standard descriptive methods were used to summarize the parameters studied. The Wilcoxon signed-rank test was used to evaluate differences between values for pain perception during PMPR (VAS, main outcome). Differences of baseline hypersensitivity levels between treatment groups were evaluated with the chi-square test (SCASS) or Wilcoxon signed-rank test (tactile hypersensitivity, VAS). All statistical tests of the hypotheses were two-sided, and a level of significance of alpha = 0.05 was employed.
Results
Baseline data
One hundred teeth in 50 subjects (31 females, 19 males) were enrolled in the study. All participants were Caucasians, aged 20–79 years (mean age ± SD = 44.84 ± 14.06). Fifty-six percent of the subjects had never smoked before, and 44 % were smokers. Thirty-six subjects showed baseline SCASS 2 on matched test and control teeth, and 14 subjects exhibited with SCASS 3 on matched test and control teeth. Six subjects with tooth category 1, 27 subjects with tooth category 2, and 17 subjects with tooth category 3 were included. There was no statistical significant difference between test and control teeth in baseline tactile hypersensitivity (VAS baseline 1.60 ± 2.09 and 1.62 ± 2.19, respectively; p = 0.617) (Table 1).
No statistically significant difference in perceived pain between the instruments used was found. For the test device Piezon Master 700 with NO PAIN technology, the average VAS value during debridement was 3.16 ± 2.10, and for the control device Mini Piezon without NO PAIN technology, this was 3.40 ± 2.59 (p = 0.490). The median was 3 for both instruments (range 1–10) (Fig. 1). Placing an arbitrary threshold for significant pain experience at the VAS score of 3 [16], 60 % (n = 30) of the subjects experienced no significant pain (VAS 0 to 3) with either instrument. Further assuming another arbitrary limit at 7 [16], two subjects perceived great pain (VAS 7 to 10) during treatment with the Piezon Master 700, and seven patients perceived great pain during treatment with the Mini Piezon (Fig. 2).
Box plot for the pain perception during debridement (median, outliers, 10, 25, 75, and 90 % percentiles). Pain perception of the instrumentation was assessed immediately after the treatment on an interval scale (visual analogue scale, VAS) ranging from 0, representing no pain or discomfort, to 10, representing maximum pain and discomfort. With a median of 3, pain perception was low for both devices
Discussion
Many studies have highlighted the importance of regular supportive periodontal therapy including PMPR. Among the most well-known studies are Hirschfield and Wasserman [17], McFall [18], Lindhe and Nyman [19], Goldman et al. [20], and Axelsson and Lindhe [21]. Indeed, there is a significant increase in tooth loss in non-compliers or irregular compliers compared to compliers [22]. Incidence of new sites with probing depth of > or =5 mm varied between 3.2 % for the compliant and 5.8 % for the non-compliant patients (mean delay from the scheduled recall sessions: compliant within 1–6 weeks; and not compliant >6 weeks) [23]. A painless treatment increases patient comfort during PMPR, and might therefore increase patient compliance [5]. This in turn may provide a better long-term prognosis for periodontal therapy.
In the present randomized-controlled and double blind trial, 100 teeth with dentin hypersensitivity were enrolled. Two teeth in each subject were matched according to air blast hypersensitivity and tooth category and were randomly assigned to debridement with an ultrasonic device with or without NO PAIN technology. Debridement of hypersensitive dentin was restricted on supragingival areas to avoid pain by gingival injury, which would have falsified the result. Pain perception of the instrumentation was recorded instantly with a VAS. Verbal reports are known to be shaped by a variety of psychosocial variables. Additionally, pain is not a simple sensory state but is influenced by cultural learning, the meaning of the situation, attention and other psychological variables [24]. Therefore, to overcome inter-individual differences between test and control patients, we investigated both devices in a split-mouth clinical trial. To overcome intra-individual differences in pain perception between different teeth, tooth categories and air blast hypersensitivity were matched between test and control teeth.
Pain perception during instrumentation was low for both ultrasonic devices (VAS median 3), and there was no statistically significant difference between the two treatment modalities. More subjects (n = 7) perceived great pain (VAS 7–10) with the Mini Piezon compared to subjects exposed to the Piezon Master 700 (n = 2) (Fisher Exact Probability Test: p = 0.159). Our results are in line with previous studies on pain perception during debridement with piezoceramic ultrasonic devices. Braun et al. compared pain intensities during debridement with hand instruments (Gracey-curettes, Hu-Friedy, Leimen, Germany), a piezo ultrasonic instrument (SirosonTMS, instrument N°3, Siemens, Bensheim, Germany) or the Vector™-system (Duerr Dental, Bietigheim-Bissingen, Germany); mean VAS values for pain perception during therapy was 3.7 ± 1.8 for the piezo ultrasonic device [25]. Kocher et al. compared pain intensities during debridement with a sonic (Sonicflex2000, KaVo, Biberach, Germany) and a piezoceramic ultrasonic scaler (PiezonMaster 400, EMS, Nyon, CH) in a split-mouth design; the median VAS was 3 for both instruments [16]. Kocher et al. concluded that the motion of the instrument’s tip might be redundant with respect to perceived pain [16]. The EMS Piezon® NO PAIN technology (Electro Medical Systems (EMS), Nyon, CH) promises minimal pain during PMPR and no injury of the gingiva, due to controlled linear oscillating instrument movements parallel to the tooth surface, combined with a sinusoidal power output and feedback control [14]. Emmelmann studied in his thesis the motion of instrument tips in an unloaded and loaded mode with a high speed camera with 7500 frames-per-second and provided first evidence that the instrument’s tip (EMS® Instrument P) of the Piezon Master 700 has a slight elliptic motion [26]. Possibly, the motion of the instrument’s tip with the Piezon Master 700 is very similar to the Mini Piezon, which is also equipped with the Piezon® technology (Fig. 3). The feedback control does not seem to have a significant effect on pain reduction in supportive periodontal therapy where little calculus is present.
Frequency distribution of VAS scores for the treatment with a the Piezon Master 700 and b the Mini Piezon (both EMS, Nyon, CH). Pain perception of the instrumentation was assessed immediately after the treatment on an interval scale (visual analogue scale, VAS) ranging from 0, representing no pain or discomfort, to 10, representing maximum pain and discomfort. Placing an arbitrary threshold at the VAS score of 3, 60 % (n = 30) of the subjects experienced no significant pain with either instrument. Further assuming another arbitrary limit at 7, two subjects perceived great pain during treatment with the Piezon Master 700, and seven patients perceived great pain during treatment with the Mini Piezon
In conclusion, both ultrasonic devices investigated in the present study showed very small pain intensities during debridement of highly hypersensitive teeth and can therefore be recommended for supportive periodontal therapy.
References
Trombelli L, Franceschetti G, Farina R (2015) Effect of professional mechanical plaque removal performed on a long-term, routine basis in the secondary prevention of periodontitis: a systematic review. J Clin Periodontol 42(Suppl 16):S221–S236. doi:10.1111/jcpe.12339
Periodontology AAo (2000) Parameter on periodontal maintenance. J Periodontol 71:849–850. doi:10.1902/jop.2000.71.5-S.849
Armitage GC, Xenoudi P (2016) Post-treatment supportive care for the natural dentition and dental implants. Periodontol 2000(71):164–184. doi:10.1111/prd.12122
Lee CT, Huang HY, Sun TC, Karimbux N (2015) Impact of patient compliance on tooth loss during supportive periodontal therapy: a systematic review and meta-analysis. J Dent Res 94:777–786. doi:10.1177/0022034515578910
Si W, Wang H, Li Q, Zhao X, Pan Y (2016) Effects of different factors influencing clinical compliance of Chinese patients with chronic periodontitis. Quintessence Int. doi:10.3290/j.qi.a35701
Crespi R, Barone A, Covani U (2005) Histologic evaluation of three methods of periodontal root surface treatment in humans. J Periodontol 76:476–481. doi:10.1902/jop.2005.76.3.476
Badersten A, Nilveus R, Egelberg J (1984) Effect of nonsurgical periodontal therapy. II Severely advanced periodontitis. J Clin Periodontol 11:63–76
Orchardson R, Collins WJ (1987) Clinical features of hypersensitive teeth. Br Dent J 162:253–256
Al-Sabbagh M, Beneduce C, Andreana S, Ciancio SG (2010) Incidence and time course of dentinal hypersensitivity after periodontal surgery. Gen Dent 58:e14–e19
Lin YH, Gillam DG (2012) The prevalence of root sensitivity following periodontal therapy: a systematic review. Int J Dent 2012:407023. doi:10.1155/2012/407023
Tammaro S, Wennstrom JL, Bergenholtz G (2000) Root-dentin sensitivity following non-surgical periodontal treatment. J Clin Periodontol 27:690–697
Brannstrom M (1963) Dentin sensitivity and aspiration of odontoblasts. J Am Dent Assoc 66:366–370
Braun A, Jepsen S, Deimling D, Ratka-Kruger P (2010) Subjective intensity of pain during supportive periodontal treatment using a sonic scaler or an Er:YAG laser. J Clin Periodontol 37:340–345. doi:10.1111/j.1600-051X.2010.01536.x
EMS -EMSS (2016) Piezon no pain built-in kit. EMS - Electro Medical Systems SA. http://new.ems-company.com/details/piezon®-no-pain-built-in-kit.html. Accessed Acces Date 2016
Petrou I, Heu R, Stranick M, Lavender S, Zaidel L, Cummins D, Sullivan RJ, Hsueh C, Gimzewski JK (2009) A breakthrough therapy for dentin hypersensitivity: how dental products containing 8 % arginine and calcium carbonate work to deliver effective relief of sensitive teeth. J Clin Dent 20:23–31
Kocher T, Rodemerk B, Fanghanel J, Meissner G (2005) Pain during prophylaxis treatment elicited by two power-driven instruments. J Clin Periodontol 32:535–538. doi:10.1111/j.1600-051X.2005.00707.x
Hirschfeld L, Wasserman B (1978) A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol 49:225–237. doi:10.1902/jop.1978.49.5.225
McFall WT Jr, Bader JD, Rozier RG, Ramsey D, Graves R, Sams D, Sloame B (1989) Clinical periodontal status of regularly attending patients in general dental practices. J Periodontol 60:145–150. doi:10.1902/jop.1989.60.3.145
Lindhe J, Nyman S (1984) Long-term maintenance of patients treated for advanced periodontal disease. J Clin Periodontol 11:504–514
Goldman MJ, Ross IF, Goteiner D (1986) Effect of periodontal therapy on patients maintained for 15 years or longer. A retrospective study. J Periodontol 57:347–353. doi:10.1902/jop.1986.57.6.347
Axelsson P, Nystrom B, Lindhe J (2004) The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol 31:749–757. doi:10.1111/j.1600-051X.2004.00563.x
Costa FO, Lages EJ, Cota LO, Lorentz TC, Soares RV, Cortelli JR (2014) Tooth loss in individuals under periodontal maintenance therapy: 5-year prospective study. J Periodontal Res 49:121–128. doi:10.1111/jre.12087
Rieder C, Joss A, Lang NP (2004) Influence of compliance and smoking habits on the outcomes of supportive periodontal therapy (SPT) in a private practice. Oral Health Prev Dent 2:89–94
Turk DC, Robinson JP (1992) Assessment of patients with chronic pain—a comprehensive approach. Guilford Press, New York, NY
Braun A, Krause F, Nolden R, Frentzen M (2003) Subjective intensity of pain during the treatment of periodontal lesions with the Vector-system. J Periodontal Res 38:135–140
Emmelmann JM (2013) Darstellung des Bewegungsmusters von in der Parodontaltherapie verwendeten Ultraschall-Instrumenten mit einer Hochgeschwindigkeitskamera. Medical University of Graz
Acknowledgments
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. We would like to thank all participants for their willingness to complete the study and Ingrid Grunert and Herbert Dumfahrt for the possibility to conduct this clinical trial at the Department of Dental Prosthetics and Restorative Dentistry, Medical University of Innsbruck, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The work was supported by the Department of Dental Prosthetics and Restorative Dentistry of the Medical University of Innsbruck, Austria.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Müller, S., Huber, H., Goebel, G. et al. Pain perception during debridement of hypersensitive teeth elicited by two ultrasonic scalers. Clin Oral Invest 21, 1559–1564 (2017). https://doi.org/10.1007/s00784-016-1971-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1971-4