Abstract
Objectives
The aim of the study was to introduce a novel three-dimensional (3D) method to quantify the relative amount of different tissue components in bone substitute-treated defects by means of integration of confocal laser imaging into micro-computed tomography (μCT) analysis.
Materials and methods
One standardized semisaddle intraosseous defect was prepared in the mandibles of six minipigs and scanned by an optical scanner to capture the surface of the fresh defect in a 3D manner. Subsequently, all the defects were filled with a biphasic calcium phosphate material. The animals were divided into two groups of three animals each, which were allowed to heal for 3 and 8 weeks, respectively. μCT analysis followed the two healing periods and was performed on all defect locations. The data from optical scanning and μCT were used for three-dimensional evaluation of bone formation, nonmineralized tissue ratio, and graft degradation. The integration of confocal laser scanning into μCT analysis through a superimposition imaging procedure was conducted using the software Amira (Mercury Computer Systems, Chelmsford, MA, USA).
Results
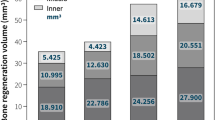
The feasibility of combining the confocal imaging into μCT data with regard to obtaining accurate 3D quantification was demonstrated. The amount of tissue components was identified and quantified in all the investigated samples. Quantitative analysis demonstrated that a significant increase in the amount of bone filling the defect was observed in vivo (p < 0.02) while a significant decrease in the amount of nonmineralized tissue occurred (p < 0.04). No difference in the amount of residual grafting material was detected between 3 and 8 weeks in vivo (p > 0.38).
Conclusions
The combination of confocal imaging and micro-computed tomography techniques allows for analysis of different tissue types over time in vivo. This method has revealed to be a feasible alternative to current bone regeneration quantification methods.
Clinical relevance
Assessment of bone formation in a large animal model is a key step in assessing the performance of new bone substitute materials. Reliable and accurate methods are needed for the analysis of the regenerative potential of new materials.




Similar content being viewed by others
References
Nkenke E, Schultze-Mosgau S, Radespiel-Tröger M, Kloss F, Neukam FW (2001) Morbidity of harvesting of chin grafts: a prospective study. Clin Oral Implants Res 12:495–502
Raghoebar GM, Louwerse C, Kalk WWI, Vissink A (2001) Morbidity of chin bone harvesting. Clin Oral Implants Res 12:503–507
Raghoebar GM, Meijndert L, Kalk WWI, Vissink A (2007) Morbidity of mandibular bone harvesting: a comparative study. Int J Oral Maxillofac Implants 22:359–365
Jensen SS, Yeo A, Dard M, Hunziker E, Schenk R, Buser D (2007) Evaluation of a novel biphasic calcium phosphate in standardized bone defects: a histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implant Res 18:752–760
Jensen SS, Bornstein MM, Dard M, Bosshardt DD, Buser D (2008) Comparative study of biphasic calcium phosphates with different HA/TCP ratios in mandibular bone defects. A long-term histomorphometric study in minipigs. J Biomed Mater Res B Appl Biomater 90:171–181
Jensen SS, Chen B, Bornstein MM, Bosshardt DD, Buser D (2011) Effect of enamel matrix derivative and parathyroid hormone on bone formation in standardized osseous defects: an experimental study in minipigs. J Periodontol 82:1197–1205
Kühl S, Brochhausen C, Götz H, Filippi A, Payer M, d'Hoedt B, Kreisler M (2012) The influence of bone substitute materials on the bone volume after maxillary sinus augmentation: a microcomputerized tomography study. Clin Oral Invest. doi:10.1007/s00784-012-0732-2
Kon K, Shiota M, Ozeki M, Yamashita Y, Kasugai S (2009) Bone augmentation ability of autogenous bone graft particles with different sizes: a histological and micro-computed tomography study. Clin Oral Implant Res 20:1240–1246
Matsumoto G, Hoshino J, Kinoshita Y, Sugita Y, Kubo K, Maeda H et al (2012) Alveolar bone regeneration using poly-(lactic acid-co-glycolic acid-co-ε-caprolactone) porous membrane with collagen sponge containing basic fibroblast growth factor: an experimental study in the dog. J Biomater Appl 27:485–493
Kühl S, Götz H, Brochhausen C, Jakse N, Filippi A, d'Hoedt B, Kreisler M (2012) The influence of substitute materials on bone density after maxillary sinus augmentation: a microcomputed tomography study. Int J Oral Maxillofac Implants 27:1541–1546
Glassman S (2009) Digital impressions for the fabrication of aesthetic ceramic restorations: a case report. Pract Proced Aesthet Dent 21:60–64
Zambon R, Mardas N, Horvath A, Petrie A, Dard M, Donos N (2012) The effect of loading in regenerated bone in dehiscence defects following a combined approach of bone grafting and GBR. Clin Oral Implant Res 23:591–601
Clozza E, Biasotto M, Cavalli F, Moimas L, Di Lenarda R (2012) Three-dimensional evaluation of bone changes following ridge preservation procedures. Int J Oral Maxillofac Implants 27:770–775
Ronay V, Sahrmann P, Bindl A, Attin T, Schmidlin PR (2011) Current status and perspectives of mucogingival soft tissue measurement methods. J Esthet Restor Dent 23:146–156
Hönig JF, Merten HA (1993) Subperiosteal versus epiperiosteal forehead augmentation with hydroxylapatite for aesthetic facial contouring: experimental animal investigation and clinical application. Aesth Plast Surg 17:93–98
Kühl S, Götz H, Hansen T, Kreisler M, Behneke A, Heil U et al (2010) Three-dimensional analysis of bone formation after maxillary sinus augmentation by means of microcomputed tomography: a pilot study. Int J Oral Maxillofac Implants 25:930–938
Schulze-Späte U, Dietrich T, Kayal RA, Hasturk H, Dobeck J, Skobe Z et al (2012) Analysis of bone formation after sinus augmentation using β-tricalcium phosphate. Compend Contin Educ Dent 33:364–368
Acknowledgments
Institut Straumann AG (Basel, Switzerland) provided the bone substitute material free of charge. No external funding, apart from the support of the authors' institution, was available for this study.
Conflict of interests
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clozza, E., Obrecht, M., Dard, M. et al. A novel three-dimensional analysis of standardized bone defects by means of confocal scanner and micro-computed tomography. Clin Oral Invest 18, 1245–1250 (2014). https://doi.org/10.1007/s00784-013-1081-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-013-1081-5