Abstract
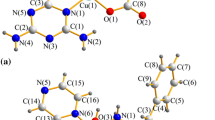
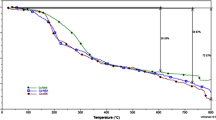
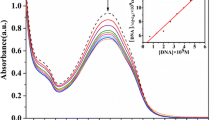
New copper(II) complexes—dimeric-[Cu(nphen)(gly)(H2O)]+ (1) and [Cu(dmphen)(gly)(NO3)(H2O)] (2) (nphen = 5-nitro-1,10-phenanthroline, dmphen = 4,7-dimethyl-1,10-phenanthroline, and gly = glycine)—have been synthesized and characterized by CHN analysis, single-crystal X-ray diffraction techniques, FTIR, EPR spectroscopy, and cyclic voltammetry. The CT-DNA-binding properties of these complexes have been investigated by thermal denaturation measurements and both absorption and emission spectroscopy. The DNA cleavage activity of these complexes has been studied on supercoiled pUC19 plasmid DNA by gel electrophoresis experiments in the absence and presence of H2O2. Furthermore, the interaction of these complexes with bovine serum albumin (BSA) has been investigated using absorption and emission spectroscopy. The thermodynamic parameters, free-energy change (ΔG), enthalpy change (ΔH), and entropy change (ΔS) for BSA + complexes 1 and 2 systems have been calculated by the van’t Hoff equation at three different temperatures (293.2, 303.2, and 310.2 K). The distance between the BSA and these complexes has been determined using fluorescence resonance energy transfer (FRET). Conformational changes of BSA have been observed using the synchronous fluorescence technique. In addition, in vitro cytotoxicities of these complexes on tumor cell lines (Caco-2, A549, and MCF-7) and healthy cells (BEAS-2B) have been examined. The antimicrobial activity of the complexes has also been tested on certain bacteria cells. The effect of mono and dimeric in the above complexes is presented and discussed.
Graphical Abstract
New copper(II) complexes—dimeric-[Cu(nphen)(gly)(H2O)]+ (1) and [Cu(dmphen)(gly) (NO3)(H2O)] (2) (nphen = 5-nitro-1,10-phenanthroline, dmphen = 4,7-dimethyl-1,10-phenanthroline and gly = glycine)—have been synthesized and characterized by CHN analysis, single-crystal X-ray diffraction techniques, FTIR and EPR spectroscopy. They have been tested for their in vitro DNA/BSA interactions by the spectroscopic methods. These complexes exhibited higher cytotoxic and antimicrobial activities. Complex 1 shows better DNA / BSA interactions in comparison to complex 2.

















Similar content being viewed by others
References
Ronconi L, Sadler PJ (2007) Coord Chem Rev 251:1633–1648
Cohen SM, Lippard SJ (2001) Prog Nucleic Acid Res Mol Biol 67:93–130
Lippert B (1999) Cisplatin: chemistry and biochemistry of a leading anticancer drug. Wiley-VCH, New York
Abu-Surrah AS, Kettunen M (2006) Curr Med Chem 13:1337–1357
Allardyce CS, Dyson PJ (2001) Platin Met Rev 45:62–69
Ott I, Gust R (2007) Arch Pharm Chem Life Sci 340:117–126
Jakupec MA, Galanski M, Arion VB, Hartinger CG, Keppler BK (2008) Dalton Trans 2:183–194
Yang P, Guo M (1999) Coord Chem Rev 185–186:189–211
Waddell J, Elvehjem CA, Steenbock H, Hart EB, Donk EV, Riising BM (1928) J Biol Chem 77:777–795
Waddell J, Steenbock H, Elvehjem CA, Hart EB, Donk EV (1929) J Biol Chem 83:251–260
Puiu SC, Zhou Z, White CC, Neubauer LJ, Zhang Z, Lange LE, Mansfield JA, Meyerhoff ME, Reynolds MM (2009) J Biomed Mater Res B Appl Biomater 91B:203–212
Schühle DT, Peters JA, Schatz J (2011) J Coord Chem Rev 255:2727–2745
Aldakov D, Bonnassieux Y, Geffroy B, Palacin S (2009) ACS Appl Mater Interfaces 1:584–589
Easmon J, Pürstinger G, Heinisch G, Roth T, Fiebig HH, Holzer W, Jäger W, Jenny M, Hofmann J (2001) J Med Chem 44:2164–2171
Chen Z, Zhang J, Zeng P, Zhang S, Jin C (2014) Z Anorg Allg Chem 640:1506–1513
Ruiz-Azuara L (1997) 07/628,628: RE 35,458, Feb. 18, United States Patent 1992
Ruiz-Azuara L (1996) 07/628,628: 5,576,326, United States Patent 1996
Ruiz-Azuara L (1992) 07/628,843: RE 35,458, Feb. 18 (1997). United States Patent 1992
Bravo-Gomez ME, Garcia-Ramos JC, Gracia-Mora I, Ruiz-Azuara L (2009) J Inorg Biochem 103:299–309
Alemon-Medina R, Brena-Valle M, Munoz-Sanchez JL, Gracia-Mora MI, Ruiz- Azuara L (2007) Cancer Chemother Pharmacol 60:219–228
Rivero-Muller A, De Vizcaya-Ruiz A, Plant N, Ruiz L, Dobrota M (2007) Chem Biol Interact 165:189–199
Trejo-Solis C, Palencia G, Zuniga S, Rodriguez-Ropon A, Osorio-Rico L, Luvia ST, Gracia-Mora I, Marquez-Rosado L, Sanchez A, Moreno-Garcia ME, Cruz A, Bravo-Gomez ME, Ruiz-Ramirez L, Rodriguez-Enriquez S, Sotelo J (2005) Neoplasia 7:563–574
Ruiz-Azuara L, Bravo-Gómez ME (2010) Curr Med Chem 17:3606–3615
Mejia C, Ruiz-Azuara L (2008) Pathol Oncol Res 14:467–472
Chikira M, Tomizawa Y, Fukita D, Sugizaki T, Sugawara N, Yamazaki T, Sasano A, Shindo H, Palaniandavar M, Antholine WE (2002) J Inorg Biochem 89:163–173
Asemave K, Yiase SG, Adejo SO, Anhwange BA (2011) Int J Inorg Bioinorg Chem 2:11–14
Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry, 5th edn. W H Freeman and Company, New York
Ottawa JH, Apps DK (1984) Biochemistry. ELBS, London
Li NC, Doody E (1952) J Am Chem Soc 74:4184–4188
Groessl M, Terenghi M, Casini A, Elviri L, Lobinski R, Dyson PJ (2010) J Anal At Spectrom 25:305–313
İnci D, Aydin R, Yılmaz D, Gençkal HM, Vatan O, Çinkılıç N, Zorlu Y (2015) Spectrochim Acta A 136(Part B):761–770
İnci D, Aydin R, Vatan O, Yılmaz D, Gençkal HM, Zorlu Y, Cavaş T (2015) Spectrochim Acta A 145:313–324
APEX2, version 2014.1-1, Bruker, 2014, Bruker AXS Inc., Madison, WI
SAINT, version 8.34A, Bruker, 2013, Bruker AXS Inc., Madison, WI
SADABS, version 2012/2, Bruker, 2012, Bruker AXS Inc., Madison, WI
SHELXTL, version 6.14, Bruker,2000, Bruker AXS Inc., Madison, WI
Spek AL (2009) Acta Cryst D65:148–155
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, Streek J (2006) J Appl Cryst 39:453–457
Reichmann ME, Rice SA, Thomas CA, Doty PJ (1954) J Am Chem Soc 76:3047–3053
Addision AW, Rao TN, Reedijk J, Rijn JV, Verschoor GC (1984) J Chem Soc Dalton Trans 7:1349–1356
Chen ZL, Jiang CF, Yan WH, Liang FP, Batten SR (2009) Inorg Chem 48:4674–4684
Castineiras A, Balboa S, Carballo R, Gonzalez-Perez JM, Niclos-Gutierrez J (2007) Z Anorg Allg Chem 633:717–723
Earney JJ, Finn CPB, Najafabadi BM (1971) J Phys C Solid St Phys 4:1013–1021
Hathaway BJ, Billing DE (1970) Coord Chem Rev 5:143–207
Dudley RJ, Hathaway BJ (1970) J Chem Soc A 12:2799–2803
Mauro ED, Domiciano SM (1999) J Phys Chem Solids 60:1849–1854
Yerli Y, Kazan S, Yalçın O, Aktas B (2006) Spectrochim Acta A Mol Biomol Spectrosc 64:642–645
Yerli Y, Köksal F, Karadag A (2003) Solid State Sci 5:1319–1323
Kivelson D, Neiman R (1961) J Chem Phys 35:149–155
Ruíz P, Ortiz R, Perelló L, Alzuet G, González-Álvarez M, Liu-González M, Sanz-Ruíz F (2007) J Biol Inorg Chem 101:831–840
Halder P, Zangrando E (2010) Polyhedron 29:434–440
Ross PD, Sabramanian S (1981) Biochemistry 20(11):3096–3102
Pyle AM, Rehmann JP, Meshoyrer R, Kumar CV, Turro NJ, Barton JK (1989) J Am Chem Soc 111(8):3051–3058
Ou ZB, Lu YH, Lu YM, Chen S, Xiong YH, Zhou XH, Mao ZW, Le XY (2013) J Coord Chem 66(12):2152–2165
Zhang S, Zhou J (2008) J Coord Chem 61(15):2488–2498
Lakshmıpraba J, Arunachalam S, Solomon RV, Venuvanalıngam P (2015) J Coord Chem 68(8):1374–1386
Inci D, Aydin R, Zorlu Y (2016) J Coord Chem 69(18):2677–2696
Ganeshpandian M, Loganathan R, Ramakrishnan S, Riyasdeen A, Akbarsha MA, Palaniandavar M (2013) Polyhedron 52:924–938
Ramadevi P, Singh R, Jana SS, Devkar R, Chakraborty D (2015) J Photochem Photobiol A Chem 305:1–10
Chetana PR, Rao R, Roy M, Patra AK (2009) Inorg Chim Acta 362:4692–4698
Rao R, Patra AK, Chetana PR (2008) Polyhedron 27:1343–1352
Rao R, Patra AK, Chetana PR (2007) Polyhedron 26:5331–5338
Wu HL, Yuan JK, Bai Y, Pan GL, Wang H, Kong J, Fan XY, Liu HM (2012) Dalton Trans 41:8829–8838
Wu HL, Yuan JK, Bai Y, Pan GL, Wang H, Shu XB (2012) J Photochem Photobiol B 107:65–72
Qian W, Gu F, Gao L, Feng S, Yan D, Liao D, Cheng P (2007) Dalton Trans 10:1060–1066
Psomas G (2008) J Inorg Biochem 102(9):1798–1811
Mohamed MS, Shoukry AA, Ali AG (2012) Spectrochim Acta Part A 86:562–570
Yanmei L, Yongheng C, Zhibin O, Shi C, Chuxiong Z, Xueyi L (2012) Chin J Chem 30:303–310
Fu X-B, Zhang J-J, Liu D-D, Gan Q, Gao H-W, Mao Z-W, Le X-Y (2015) J Inorg Biochem 143:77–87
Terenzi A, Tomasello L, Spinello A, Bruno G, Giordano C, Barone G (2012) J Inorg Biochem 117:103–110
Meyer-Almes FJ, Porschke D (1993) Biochemistry 32(16):4246–4253
Karlin KD, Cohen BI, Hayes JC, Farooq A (1987) Zubieta. J Inorg Chem 26:147–153
Lee M, Rhodes AL, Wyatt MD, Forrow S, Hartley JA (1993) Biochemistry 32:4237
Lakowicz JR, Weber G (1973) Biochemistry 112(21):4161–4170
Subbaraj P, Ramu A, Raman N, Dharmaraja J (2014) J Coord Chem 67:2747–2764
Cory M, McKee DD, Kagan J, Henry DW, Miller JA (1985) J Am Chem Soc 107:2528–2536
Kelly JM, Tossi AB, McConnell DJ, OhUigin C (1985) Nucleic Acids Res 13(17):6017–6034
Goswami TK, Gadadhar S, Karande AA, Chakravarty AR (2013) Polyhedron 52:1287–1298
Seng HL, Tan KW, Maah MJ, Tan WT, Hamada H, Chikira M, Ng CH (2009) Polyhedron 28:2219–2227
Katwal R, Kaur H, Kapur BK (2013) Sci Revs Chem Commun 3(1):1–15
Powell DH, Helm L, Merbach AE (1991) J Chem Phys 95:9258–9265
Schwenk CF, Rode BM (2003) Chem Phys Chem 4:931–943
Cowan JA (2001) Curr Opin Chem Biol 5:634–642
Branum ME, Tipton AK, Zhu S, Que L Jr (2001) J Am Chem Soc 123:1898–1904
Sreedhara A, Freed JD, Cowan JA (2000) J Am Chem Soc 122:8814–8824
Deal KA, Burstyn JN (1996) Inorg Chem 35:2792–2798
Hegg EL, Burstyn JN (1998) Coord Chem Rev 173:133–165
Kenely RA, Fleming RH, Laine RM, Tse D, Winterle JS (1984) Inorg Chem 23:1870–1876
Chin J, Banaszezyk M, Jubian V, Zou XJ (1989) J Am Chem Soc 111:186–190
Chin J, Zou X (1988) J Am Chem Soc 110:223–225
Gillespie P, Ramirez F, Ugi I, Marquarding D (1993) Angew Chem Int Ed 12:91
Westheimer FH (1968) Acc Chem Res 1:70–78
Sigman DS (1990) Biochemistry 29:9097–9105
Reddy PR, Raju N (2012) Polyhedron 44:1–10
Reddy PR, Shilpa A, Raju N, Raghavaiah P (2011) J Inorg Biochem 105:1603–1612
Reddy PR, Shilpa A (2012) Chem Biodivers 9:2262–2281
Sigman DS (1986) Acc Chem Res 19(6):180–186
Hawkins MJ, Soon-Shiong P, Desaia N (2008) Adv Drug Delivery Rev 60:876–885
Paramaguru G, Kathiravan A, Selvaraj S, Venuvanalingam P, Renganathan R (2010) J Hazard Mater 175:985–991
Du N, Sheng L, Liu Z, Hu X, Xu H, Chen S (2013) Chem Cent J 7:97
Zhang LN, Wu FY, Liu AH (2011) Spectrochim Acta A 79:97–103
Lakowitcz JR (1999) Principles of fluorescence spectroscopy. Plenum Press, New York, p 237
Ware WR (1962) J Phys Chem 66:455–458
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, New York, p 277
Hua YJ, Liu Y, Wang JB, Xiao XH, Qu SS (2004) J Pharm Biomed Anal 36:915–919
Gao H, Lei LD, Liu JQ, Kong Q, Chen XG, Hu ZD (2004) J Photochem Photobiol Part A 167:213–221
Lunardi CN, Tedesco AC, Kurth TL, Brinn IM (2003) J Photochem Photobiol Sci 2:954–959
Wang TH, Zhao ZM, Wei BZ, Zhang L, Ji L (2010) J Mol Struct 970(1–3):128–133
Tian MY, Zhang XF, Xie L, Xiang JF, Tang YL, Zhao CQ (2008) J Mol Struct 892(1–3):20–24
Wang F, Huang W, Dai ZX (2008) J Mol Struct 875(1–3):509–514
Skaikh SMT, Seetharamappa J, Kandagal PB, Ashoka S (2006) J Mol Struct 786:46–52
Cheng XX, Lui Y, Zhou B, Xiao XH, Liu Y (2009) Spectrochim Acta A 72:922–928
Miller JN (1979) Proc Anal Div Chem Soc 16(3):203–208
Guo M, Lue WJ, Li MH, Wang W (2008) Eur J Med Chem 43(10):2140–2148
Zhang G, Wang Y, Zhang H, Tang S, Tao W (2007) Pestic Biochem Physiol 87(1):23–29
Guo XJ, Sun XD, Xu SK (2009) J Mol Struct 931(1–3):55–59
Xiao Q, Huang S, Liu Y, Tian FF, Zhu JC (2009) J Fluoresc 19(2):317–326
Rizzotto M (2012) A search for antibacterial agents. Bobbarala V (ed) Chapter 5, p 73
Obaleye JA, Tella AC, Bamigboye MO (2012) A search for antibacterial agents. Bobbarala (ed) Chapter 10, p 198
Liu H, Yang W, Zhou W, Xu Y, Xie J, Li M (2013) Inorg Chim Acta 405:387–394
Acknowledgements
We thank the Research Fund of Uludag University for the financial support given to the research projects (Project Numbers OUAP (F)-2015/14 and KUAP(F)-2012/76) and the Scientific and Technological Research Council of Turkey (TUBITAK) for Domestic PhD Scholarship intended for Priority Areas of the first author (Code: 2211-C). This study is a part of doctoral thesis in progress of the first author at the Graduate School of Natural and Applied Sciences of Uludag University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
İnci, D., Aydın, R., Vatan, Ö. et al. Synthesis and crystal structures of novel copper(II) complexes with glycine and substituted phenanthrolines: reactivity towards DNA/BSA and in vitro cytotoxic and antimicrobial evaluation. J Biol Inorg Chem 22, 61–85 (2017). https://doi.org/10.1007/s00775-016-1408-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-016-1408-1