Abstract
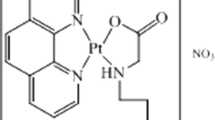
Three new water-soluble tungstenocene derivatives were synthesized and characterized using 3-hydroxy-4-pyrone ligands, which provide aqueous stability to the complexes. The antiproliferative activities of the complexes on HT-29 colon cancer and MCF-7 breast cancer cell lines were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and showed the new tungstenocene derivatives have higher antiproliferative action than tungstenocene dichloride (Cp2WCl2, where Cp is cyclopentadienyl). The binding interactions of the tungstenocenes with human serum albumin (HSA) were investigated using fluorescence spectroscopy and molecular modeling methods. Analysis of the fluorescence quenching spectra indicates that the tungstenocene complexes bind HSA by hydrophobic interactions and hydrogen bonding at fatty acid binding site 6 and drug binding site II. Docking studies provided a description of the hydrophobic interactions and hydrogen bonding by which the tungstenocenes become engaged with HSA. It was determined that the binding affinity of the tungstenoecenes for HSA is in the order Cp2WCl2 < [Cp2W(ethyl maltolato)]Cl < [Cp2W(maltolato)]Cl < [Cp2W(kojato)]Cl, consistent with the hydrophobic interactions and the number of hydrogen bonds involved.
Graphical abstract









Similar content being viewed by others
References
Jaouen G (ed) (2006) Bioorganometallics. Wiley, Weinheim
Jaouen G, Metzler-Nolte N (eds) (2010) Medicinal Organometallic Chemistry. Springer, Heidelberg
Gasser G, Ott I, Metzler-Nolte N (2011) J Med Chem 54:3
Köpf H, Köpf-Maier P (1979) Angew Chem Int Ed Engl 18:477
Köpf-Maier P (1994) Eur J Clin Pharmacol 47:1
Köpf-Maier P, Köpf H (1988) Struct Bond 70:103
Köpf-Maier P, Köpf H (1987) Chem Rev 87:137
Köpf-Maier P, Köpf H (1994) In: Fricker SP (ed) Metal Compounds in Cancer Therapy, Organometallic Titanium, Vanadium, Niobium, Molybdenu and Rhenium Complexes - Early Transition Metal Antitumor Drugs. Chapman and Hall, London, pp 109–146
Harding MM, Mokdsi G (2000) Curr Med Chem 7:1289
Meléndez E (2002) Crit Rev Oncol Hematol 42:309
Abeysinghe PM, Harding MM (2007) Dalton Trans 32:3472
Meléndez E (2012) J Organomet Chem 706–707:4
Berdel WE, Schmoll H-J, Scheulen ME, Korfel A, Knoche MF, Harstrick A, Bach F, Baumgart J, Saβ G (1994) J Cancer Res Clin Oncol 120(Supp):R172
Korfel A, Scheulen ME, Schmoll H-J, Gründel O, Harstrick A, Knoche M, Fels LM, Skorzec M, Bach F, Baumgart J, Saβ G, Seeber S, Thiel E, Berdel W (1998) Clin Cancer Res 4:2701
Luemmen G, Sperling H, Luboldt H, Otto T, Ruebben H (1998) Cancer Chemother Pharmacol 42:415
Christodoulou CV, Ferry DR, Fyfe DW, Young A, Doran J, Sheehan TMT, Eliopoulos A, Hale K, Baumgart J, Sass G, Kerr DJ (1998) J Clin Oncol 16:2761
Kröger N, Kleeberg UR, Mross K, Sass G, Hossfeld DK (2000) Onkologie 23:60
Baumgart J, Berdel WE, Fiebig H, Unger C (2000) Onkologie 23:576
Kopf-Maier P (1980) J Inorg Nucl Chem 42:1789
Campell KS, Dillon CT, Smith SV, Harding MM (2007) Polyhedron 26:456
Feliciciano I, Matta J, Meléndez E (2009) J Biol Inorg Chem 14:1109
Acevedo-Acevedo D, Matta J, Meléndez E (2011) J Organomet Chem 696:1032
Waern JB, Dillon CT, Harding MM (2005) J Med Chem 48:2093
Gleeson B, Claffey J, Hogan M, Müller-Bunz H, Wallis D, Tacke M (2010) Inorg Chim Acta 363:1831
Ahmet MT, Frampton CS, Silver J (1988) J Chem Soc Dalton Trans 1159
Finnegan MM, Rettig SJ, Orvig C (1986) J Am Chem Soc 108:5033
Nelson WO, Karpishin TB, Rettig SJ, Orvig C (1988) Inorg Chem 27:1045
Yu P, Phillips BL, Olmstead MM, Casey WH (2002) J Chem Soc Dalton Trans 2119
Saatchi K, Thompson KH, Patrick BO, Pink M, Yuen VG, McNeill JH, Orvig C (2005) Inorg Chem 44:2689
Thompson KH, McNeill JH, Orvig C (1999) Chem Rev 99:2561
Vera JL, Román FR, Meléndez E (2006) Biorg Med Chem 14:8683
Kotz JC, Vining W, Coco W, Rosen R, Dias AR, Garcia MH (1983) Organometallics 2:68
Mossman T (1983) J Immunol Methods 65:55
Denizot F, Lang R (1986) J Immunol Methods 89:271
Köpf-Maier P, Köpf H (1984) J Cancer Res Clin Oncol 108:336
Vera J, Gao LM, Santana A, Matta J, Meléndez E (2011) Dalton Trans 40:9957
Vessières A, Top S, Beck W, Hillard E, Jaouen G (2006) Dalton Trans 529
Vessières A, Plamont M-A, Cabestaing C, Claffey J, Dieckmann S, Hogan M, Müller-Bunz H, Strohfeldt K, Tacke M (2009) J Organomet Chem 694:874
Top S, Kaloun EB, Vessières A, Laïos I, Leclercq G, Jaouen G (2002) J Organomet Chem 643:350
Dugaiczyk A, Law SW, Dennison OE (1982) Proc Natl Acad Sci USA 79:71
He XM, Carter DC (1992) Nature 358:209
Tang J, Liam N, He X, Zhang G (2008) J Mol Struct 889:408
Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K (1999) Protien Eng 12:439
Lu Y, Feng Q, Cui F, Xing W, Zhang G, Yao X (2010) Bioorg Med Chem Lett 20:6899
Sułkowska A, Bojko B, Rownicka J, Sułkowski WW (2006) J Mol Struct 792–793:249
Tian J, Liu J, Xie J, Yao X, Hu Z, Chen X (2004) J Photochem Photobiol B Biolo 74:39
Guo X, Sun X, Xu S (2009) J Mol Struct 931:55
Cui F, Fan J, Lib J, Hu Z (2004) Bioorg Med Chem 12:151
Lakowicz J (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, New York
Huang YB, Liu BZ, Yu Z, Gao XY, Zi YQ (2010) J Lumin 130(3):360
Ross P, Subramanian S (1981) Biochemistry 20:3096
Zhang G, Ma Y, Wang L, Zhang Y, Zhou J (2012) Food Chem 133:264
Curry S (2011) In: Otagiri M (ed) Human serum albumin—new insights on its structural dynamics, functional impacts and pharmaceutical applications. Sojo University Publishing Center, Kumamoto, pp 1–20
Tripos International SYBYL 8.1. Tripos International, St Louis
Jain AN (2003) J Med Chem 46:499
Sarsam SW, Nutt DR, Strohfeldt K, Watson KA (2011) Metallomics 3:152
Frisch MJ et al (2003) Gaussian 03, revision B.04. Gaussian, Pittsburgh
Acknowledgments
E.M. acknowledges the NIH-MBRS SCORE program at the University of Puerto Rico Mayagüez for financial support via NIH-MBRS-SCORE program grant S06 GM008103-37. We are grateful to Jesús Olivero-Verbel from the University of Cartagena, Colombia, for allowing us to use the program SYBIL and for revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Domínguez-García, M., Ortega-Zúñiga, C. & Meléndez, E. New tungstenocenes containing 3-hydroxy-4-pyrone ligands: antiproliferative activity on HT-29 and MCF-7 cell lines and binding to human serum albumin studied by fluorescence spectroscopy and molecular modeling methods. J Biol Inorg Chem 18, 195–209 (2013). https://doi.org/10.1007/s00775-012-0964-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-012-0964-2