Abstract
The inositol phosphates are ubiquitous metabolites in eukaryotes, of which the most abundant are inositol hexakisphosphate (InsP 6) and inositol 1,3,4,5,6-pentakisphosphate [Ins(1,3,4,5,6)P 5)]. These two compounds, poorly understood functionally, have complicated complexation and solid formation behaviours with multivalent cations. For InsP 6, we have previously described this chemistry and its biological implications (Veiga et al. in J Inorg Biochem 100:1800, 2006; Torres et al. in J Inorg Biochem 99:828, 2005). We now cover similar ground for Ins(1,3,4,5,6)P 5, describing its interactions in solution with Na+, K+, Mg2+, Ca2+, Cu2+, Fe2+ and Fe3+, and its solid-formation equilibria with Ca2+ and Mg2+. Ins(1,3,4,5,6)P 5 forms soluble complexes of 1:1 stoichiometry with all multivalent cations studied. The affinity for Fe3+ is similar to that of InsP 6 and inositol 1,2,3-trisphosphate, indicating that the 1,2,3-trisphosphate motif, which Ins(1,3,4,5,6)P 5 lacks, is not absolutely necessary for high-affinity Fe3+ complexation by inositol phosphates, even if it is necessary for their prevention of the Fenton reaction. With excess Ca2+ and Mg2+, Ins(1,3,4,5,6)P 5 also forms the polymetallic complexes [M4(H2L)] [where L is fully deprotonated Ins(1,3,4,5,6)P 5]. However, unlike InsP 6, Ins(1,3,4,5,6)P 5 is predicted not to be fully associated with Mg2+ under simulated cytosolic/nuclear conditions. The neutral Mg2+ and Ca2+ complexes have significant windows of solubility, but they precipitate as [Mg4(H2L)]·23H2O or [Ca4(H2L)]·16H2O whenever they exceed 135 and 56 μM in concentration, respectively. Nonetheless, the low stability of the [M4(H2L)] complexes means that the 1:1 species contribute to the overall solubility of Ins(1,3,4,5,6)P 5 even under significant Mg2+ or Ca2+ excesses. We summarize the solubility behaviour of Ins(1,3,4,5,6)P 5 in straightforward plots.
Similar content being viewed by others
Introduction
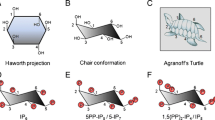
The myo-inositol phosphates (InsPs) form a broad panel of eukaryotic-specific signalling metabolites that, except for inositol 1,4,5-trisphosphate, are still poorly understood (reviewed in [1]). Together with inositol hexakisphosphate (InsP 6), inositol 1,3,4,5,6-pentakisphosphate [Ins(1,3,4,5,6)P 5, Fig. 1] is the most abundant member of the family [2–5]. Ins(1,3,4,5,6)P 5 is the metabolic precursor of InsP 6 [6], and both Ins(1,3,4,5,6)P 5 and InsP 6 are precursors of inositol pyrophosphates (reviewed in [7]). Mice that lack the enzyme responsible for the synthesis of Ins(1,3,4,5,6)P 5 die during embryonic development, indicating an essential role for this compound and/or its derived metabolites [8]. However, the function of Ins(1,3,4,5,6)P 5 is not yet clear. Ins(1,3,4,5,6)P 5, as well as other higher InsPs, can clearly bind to pleckstrin homology (PH) domains, thus competitively inhibiting their interactions with phosphoinositide headgroups [9]. Although in most in vitro assays various higher InsPs will act on a given PH-domain-containing protein, the discovery that the “minor” isomer inositol 1,2,3,5,6-pentakisphosphate is the specific ligand of pleckstrin gives credence to the idea that in vivo specific InsPs target specific PH domains [10]. However, no interaction with a PH domain that is clearly specific to Ins(1,3,4,5,6)P 5 has been reported to date. An apparently specific activity of Ins(1,3,4,5,6)P 5 has been uncovered in the Wnt/β-catenin pathway, in which the compound accumulates in response to Wnt3a and mediates the stabilization of β-catenin [11]; the direct protein target of Ins(1,3,4,5,6)P 5 in this pathway is not known. Meanwhile, Ins(1,3,4,5,6)P 5 fulfils what is probably a different function altogether in certain non-mammalian erythrocytes that contain very high concentrations of the compound (reviewed in [1]).
Elucidation of the biological roles of the higher InsPs has been complicated by their intricate structural and metabolic interrelatedness. This has been aggravated by the unusual and often non-intuitive behaviour displayed by these highly charged compounds, in particular in the presence of multivalent cations. This behaviour can be the source of many artefacts (reviewed in [12]). For InsP 6, we have over the past few years strived to make a rigorous and at the same time “biological-user-friendly” description of its chemistry with multivalent cations [13–15]. We have also studied the chemistry of inositol 1,2,3-trisphosphate [Ins(1,2,3)P 3], focusing on the likelihood of its proposed interaction with Fe3+ in the cellular context [16]. In the present paper we study Ins(1,3,4,5,6)P 5, focusing on its interactions with Na+, K+, Ca2+, Mg2+, Cu2+, Fe2+ and Fe3+ in solution, and its solid-formation equilibria with Ca2+ and Mg2+. Our results predict that Ins(1,3,4,5,6)P 5 does not have a high enough affinity for Mg2+ to be fully associated with this cation under cytosolic and nuclear conditions. They also predict that the compound would be fully soluble under those conditions, and that it even has a significant window of solubility under calcium-rich conditions such as those of the extracellular medium. We have summarized the non-intuitive solubility behaviour of Ins(1,3,4,5,6)P 5 in straightforward plots that should be of help when planning experiments with this compound.
Materials and methods
Chemicals
All common laboratory chemicals were of reagent grade, purchased from commercial sources and used without further purification. NaCl, KCl, CaCl2·2H2O, MgCl2·6H2O, CuSO4·5H2O, (NH4)2Fe(SO4)2·6H2O, and Fe(ClO4)3·xH2O were used as metal sources. Solutions of the metals were standardized according to standard techniques [17]. Ins(1,3,4,5,6)P 5 was obtained from myo-inositol in 86% isolated overall yield over five steps. Briefly, transesterification of myo-inositol with trimethyl orthobenzoate in the presence of an acid catalyst followed by acid hydrolysis of the product, myo-inositol 1,3,5-orthobenzoate, gave 2-O-benzoyl myo-inositol, which was then phosphorylated. Deprotection of the fully protected pentakisphosphate followed by the removal of the benzoate ester in concentrated aqueous ammonia afforded Ins(1,3,4,5,6)P 5 as the hexaammonium salt, (NH4)6H4L, which was verified by elemental analysis [Anal. calc. (%) for C6H35P5O21N6: C 10.6, H 5.2, N 12.3. Found (%): C 10.3, H 5.4, N 12.4.] to conform to the previous formula [18]. Solutions of Ins(1,3,4,5,6)P 5 were prepared by weighing this hexaammonium salt (NH4)6H4L. The standard HCl solutions were prepared from Merck standard ampoules. The titrant solution [0.1 M solution of Me4N(OH) in 0.15 M Me4NCl] was prepared by dissolving Me4N(OH)·5H2O (Fluka), and was standardized with potassium biphthalate.
IR spectroscopy, elemental analysis and thermal analysis
IR spectroscopy was carried out with a Bomen Fourier transform IR spectrophotometer, with samples present as 1% KBr pellets. Elemental analysis (C, H) was performed using a Carlo Erba EA 1108 instrument. The cacium content in the solid samples was determined gravimetrically as previously described [13]. Magnesium was determined volumetrically according to standard techniques [17]. Thermal analysis was performed with a Shimadzu DTA-50, TGA-50 instrument with a TA 50I interface, using a platinum cell and nitrogen atmosphere. Experimental conditions were 1 °C min−1 temperature ramp rate and 50 mL min−1 nitrogen flow rate.
Potentiometric measurements
All solutions were freed of carbon dioxide by argon bubbling. The protonation constants of Ins(1,3,4,5,6)P 5 were determined at 37.0 °C, 0.15 M ionic strength in the non-interacting electrolyte Me4NCl. Five potentiometric titrations, comprising about 150 experimental points each, were carried out in the Ins(1,3,4,5,6)P 5 concentration interval 0.5–3 mM, covering pH values between 2 and 11.
Since an ammonium salt was used as the Ins(1,3,4,5,6)P 5 source, the (weak) acidity of the ammonium ion had to be taken into account when the desired constants were calculated. Thus, the acid dissociation constant of ammonium was determined de novo under the conditions of the study, through three potentiometric titrations using NH4Cl. The hydrolysis constants of Fe(III) under the same conditions were taken from data previously reported [14].
Then the behaviour of Ins(1,3,4,5,6)P 5 in the presence of Na+, K+, Ca2+, Mg2+, Cu2+, Fe2+ and Fe3+ ions was analysed, also in 0.15 M Me4NCl and at 37.0 °C. Three to eight potentiometric titrations were carried out for each cation (about 150 experimental points for each titration), at metal ion concentrations ranging from 1 to 50 mM (for alkali metal ions) or 0.5 to 3 mM (for alkaline earth and transition metal ions), and Ins(1,3,4,5,6)P 5 to metal molar ratios from 0.01 to 2 (for alkali metal ions) and from 0.2 to 3 (for alkaline earth and transition metal ions). Owing to the lower affinity towards Ins(1,3,4,5,6)P 5 expected for M+ cations in comparison with M2+ or M3+, higher absolute concentrations of metal ions and lower Ins(1,3,4,5,6)P 5 to metal molar ratios were used in the former cases. Potentiometric titrations were carried out as previously described [14], I being adjusted by addition of Me4NCl.
The cell constants, E°, and the liquid junction potentials were determined under the same conditions using the computer program GLEE [19]. The data obtained were analysed using the HYPERQUAD program [20]. In all cases, the fit of the values predicted by the model to the experimental data was estimated on the basis of the parameter σ corresponding to the scaled sum of square differences between predicted and experimental values. Then, the constants were used to produce species distribution diagrams using the HySS program [21].
Synthesis of [Mg4(H2L)]·23H2O and [Ca4(H2L)]·16H2O
An 8.8 mM aqueous solution of Ins(1,3,4,5,6)P 5 was prepared, and the pH was adjusted to 10–11 by addition of 1 M LiOH. To 5 mL (0.044 mmol) of this solution, 36.0 mg of MgCl2·6H2O (0.18 mmol) dissolved in the minimum amount of water was added. A white solid immediately appeared, and was separated by centrifugation, washed with water (2 × 5 mL), and dried with ethanol (1 × 5 mL). The preparation of [Ca4(H2L)]·16H2O followed a similar procedure, starting from 26.0 mg of CaCl2·2H2O (0.18 mmol) as the metal source. The yield was 63% for [Mg4(H2L)]·23H2O and 56% for [Ca4(H2L)]·16H2O. Anal. Calc. for Mg4C6H55O44P5: C 6.7, H 5.1, Mg 9.0%. Found: C 6.3, H 5.0, Mg 9.5%. Anal. Calc. for Ca4C6H41O37P5: C 7.1, H 4.1, Ca 15.7%. Found: C 6.8, H 4.0, Ca 15.2%. The thermal analysis agreed with the proposed formula: 38.4% weight loss for [Mg4(H2L)]·23H2O and 28.1% weight loss for [Ca4(H2L)]·16H2O, corresponding to the elimination of water, compared with calculated values of 38.2% for [Mg4(H2L)]·23H2O and 28.2% for [Ca4(H2L)]·16H2O.
Solubility measurements
Solubility measurements were carried out at constant ionic strength, I = 0.15 M Me4NCl, and 37.0 °C. An amount of 10–40 mg of the compound—[Mg4(H2L)]·23H2O or [Ca4(H2L)]·16H2O)—was suspended in 10.0 mL of 0.15 M aqueous Me4NCl at 37.0 °C. Known amounts of HCl were added, so as to reach equilibrium points corresponding to measurable amounts of metal ion in solution. Each mixture was kept in a glass jacketed cell under continuous stirring until the measured pH was constant (about 1 week). After the equilibrium had been reached, the solid in excess was filtered out (Macherey-Nagel MN 640 m paper), and the total metal ion concentration was determined in the supernatant. Total calcium and magnesium contents were determined volumetrically according to standard techniques [17]. With these M(II) concentration values, and with the assumption of a 4:1:2 stoichiometry [M(II)/Ins(1,3,4,5,6)P 5/H+], total amounts of Ins(1,3,4,5,6)P 5 in the solution were calculated. Then total concentrations of M(II), Ins(1,3,4,5,6)P 5 and H+ were used as inputs for the HySS program [21] to determine the equilibrium concentrations of (free) M2+ and H2L8−, which define the K s0. In this calculation, the complete set of solution equilibria previously measured were taken into account. At least three independent determinations were performed for each metal ion.
Results and discussion
Protonation equilibria of Ins(1,3,4,5,6)P 5
Ins(1,3,4,5,6)P 5 can be considered as a polyprotic acid containing ten protons, H10L. The values for the corresponding deprotonation/protonation equilibrium constants, which are needed for studying the metal complexation reactions, are presented in Table 1. We were able to detect the first seven of these protonation reactions, the last three not being amenable to our methods, owing to the strong acidity of the species H10L, H9L− and H8L2− (which should have pK a values smaller than 1.1). The log β values determined followed the expected order and are consistent with values in a previous report [22] (in which only five protonation constants that had measured by potentiometry, in 0.2 M KCl and at 37.0 °C, were reported). A species distribution diagram for Ins(1,3,4,5,6)P 5 in the absence of metal ions is shown in Fig. 2. For neutral pH values, the predominant species are H4L6− and H3L7−.
Interactions between Ins(1,3,4,5,6)P 5 and alkali metal ions
Although the interactions with the alkali metal ions are expected to be very weak, it is important to describe them, as either Na+ or K+ cations are present in biological media in a large excess with respect to Ins(1,3,4,5,6)P 5. The stability constants for the Na(I)–Ins(1,3,4,5,6)P 5 and K(I)–Ins(1,3,4,5,6)P 5 species (Table 2), although exceptionally high for complexes involving alkali metal ions, are not high in absolute terms. Figure 3 shows species distribution diagrams for 1 mM Ins(1,3,4,5,6)P 5 in the presence of 100-fold excess K+ (a similar graph for Na+ is presented in Fig. S1). Under these conditions (which are close to those of part of the experimental titrations), metal-containing species become detectable only above pH 7. In contrast, for 1 mM Ins(1,3,4,5,6)P 5 and 1 mM M+, the percentage of the ligand associated to either ion is negligible (speciation plots not shown). The comparison between Na+ and K+, in terms of the formation constants (Table 2) or of species distribution diagrams (Figs. 3 and S1), shows that Ins(1,3,4,5,6)P 5 interacts with these two ions in very similar ways.
Interactions in solution between Ins(1,3,4,5,6)P 5 and divalent ions and Fe3+
Table 2 lists the equilibrium constants for the interactions of Ins(1,3,4,5,6)P 5 with divalent cations in 0.15 M Me4NCl. The high charge displayed by Ins(1,3,4,5,6)P 5 species in the absence of interacting cations (Fig. 2) indicates that strong electrostatically driven interactions with divalent cations must be expected; this is confirmed by the values of the stability constants. In addition to species with ligand-to-metal 1:1 stoichiometry, which were observed throughout the pH range studied, the neutral complexes [M4(H2L)] and [M5L] were detected, for alkaline earth and transition metal ions, respectively. Figure 4a shows the species distribution diagram for Ca2+, with 1 mM Ins(1,3,4,5,6)P 5 and 1 mM Ca2+. At pH values around 7.4, 50% of the ligand is still free, even though there is a significant amout of the species [Ca4(H2L)]. The presence of excess Ins(1,3,4,5,6)P 5 in the system changes the speciation diagram slightly, giving rise to lower concentrations of free Ca2+ at acidic pH values (plot not shown). The strengths of the interactions with Ca2+ and Mg2+ are similar; accordingly, the species distribution diagrams for these two cations are similar [Fig. 4, plots given for 1 mM Ins(1,3,4,5,6)P 5 and 1 mM M2+]. Although, over the range of divalent cations studied, the interaction with Ins(1,3,4,5,6)P 5 is fairly independent of the particular cation (Table 2), slight differences are observed. In particular, the transition metal ions display higher stability constants than the alkaline earth ones, as reflected in the speciation diagrams given in Figs. 4, S2 and S3 for 1 mM Ins(1,3,4,5,6)P 5 and 1 mM M2+. For example, the amount of M2+ left free at pH 7.4 is significant for the alkaline earth metal ions (Fig. 4), but is negligible for the transition metal ones (Figs. S2, S3 for Cu2+ and Fe2+, respectively).
In comparison with the divalent cations, Fe3+ forms much more stable complexes with Ins(1,3,4,5,6)P 5. The higher values of the stability constants (Table 2) are reflected in the species distribution diagram (Fig. 5), in which the highly deprotonated species [Fe(HL)]6− predominates at pH 7.4. The higher charge of the central atom in comparison with the divalent cations gives rise to stronger interactions and consequently to a higher extent of ligand deprotonation.
Comparative coordination ability of InsP 6, Ins(1,3,4,5,6)P 5 and Ins(1,2,3)P 3
During the last few years, we have reported quantitative data on the interaction of InsP 6, Ins(1,2,3)P 3 and now Ins(1,3,4,5,6)P 5 with metal ions [13–16]. It is therefore possible to compare the metal complexation behaviours of these three InsPs. A direct comparison of the stability constants is not straightforward because of the different complex species formed and the different degrees of protonation that the ligands can exhibit. Nonetheless, the overall strengths of interaction can be compared in terms of the quotient between bound ligand and unbound ligand. This quotient represents the fraction of all complex species containing InsP and metal relative to all forms of the free ligand. Taking K+ for example, the complexes formed with InsP x are [K3(H4L)]5−, [K4(H3L)]5−, [K5(H2L)]5− and [K6L]6− for InsP 6, [K4L]6−, [K3(HL)]6− and [K2(H2L)]6− for Ins(1,3,4,5,6)P 5 and [KL]5−, [K(HL)]4−, [K(H2L)]3− and [K2(H4L)] for Ins(1,2,3)P 3. So the quotients, Q, are calculated as follows:
The log Q values for three ions, K+, Mg2+ and Fe3+, are plotted in Fig. 6 as a function of pH, for 1 mM cation and ligand. Their values increase sharply with cation charge (from K+ to Fe3+), as expected of predominantly electrostatic interactions.
Comparative behaviour of inositol 1,2,3-trisphosphate [Ins(1,2,3)P 3], Ins(1,3,4,5,6)P 5 and inositol hexakisphosphate (InsP 6). The logarithms of the quotients between bound ligand and unbound ligand are plotted versus pH. Other conditions were 0.15 M Me4NCl, 37.0 °C, 1 mM Ins(1,2,3)P 3, 1 mM Ins(1,3,4,5,6)P 5, 1 mM InsP 6, 1 mM K+, 1 mM Mg2+ and 1 mM Fe3+. The equilibrium constants used were taken from this and previous reports [13–16]
With respect to K+, Ins(1,2,3)P 3 exhibits the strongest interaction at any pH value under the conditions studied (Fig. 6). This is possible because under 1:1 metal–ligand conditions, polymetallic species (which are formed by Ins(1,3,4,5,6)P 5 and InsP 6 but not by Ins(1,2,3)P 3 except for [K2(H4L)] under acidic conditions) are quantitatively irrelevant. Under metal-excess conditions (100:1), which favour coordinative interactions in complexes between Ins(1,3,4,5,6)P 5 or InsP 6 and two to six K+ ions, the scenario changes, with InsP 6 displaying the strongest interaction above pH 8 (Fig. S4).
The strongest interaction towards Mg2+ is clearly established by InsP 6, as expected from the fact that it is the most highly charged of the three compounds at any pH value. InsP 6 forms with Mg2+, in addition to 1:1 complexes, the neutral species [Mg5(H2L)], analogous to the neutral [Mg4(H2L)] complex now described for Ins(1,3,4,5,6)P 5 (Table 2). Under conditions of metal excess, the formation of such polymetallic complexes can be expected to shift the relative strengths of interaction in favour of both InsP 6 and Ins(1,3,4,5,6)P 5 with respect to Ins(1,2,3)P 3; however, the occurrence of precipitation in this range (see below) makes it difficult to draw meaningful plots analogous to that in Fig. 6.
The interaction with Fe3+ is very strong for the three InsPs. The log Q values are high and increase in very similar ways (from about 3 to about 7) between pH 4 and 7, while above pH 7, Ins(1,3,4,5,6)P 5 becomes a more effective Fe3+ chelator than the other two InsPs, especially in comparison with InsP 6. These results are unexpected, because, unlike Ins(1,2,3)P 3 and InsP 6, Ins(1,3,4,5,6)P 5 lacks the 1,2,3-trisphosphate motif usually believed to be necessary and sufficient for high-affinity complexation of this cation by InsPs. In derivatives of myo-inositol, the substituent at C-2 is the only axial one (see Fig. 1), and therefore the 1,2,3-trisphosphate motif is unique in having three phosphates in a cis relationship to one another. This motif is thought to flip to the normally unfavourable axial–equatorial–axial disposition upon the complexation of Fe3+ [23]. In contrast, Ins(1,3,4,5,6)P 5 has all its phosphates in the equatorial form (and it is highly unlikely to adopt a thermodynamically unfavourable all-axial conformation). The evidence for the importance of the 1,2,3-trisphosphate motif comes from assays in which the InsPs prevent the iron-catalysed generation of hydroxyl radical through the Fenton reaction. All InsPs tested so far that contain the 1,2,3-trisphosphate motif, including InsP 6 and Ins(1,2,3)P 3, are highly effective at preventing hydroxyl radical formation, while those lacking this motif, including Ins(1,3,4,5,6)P 5 in particular, are clearly less effective [24, 25]. Our results thus indicate that high-affinity complexation of Fe3+ by InsPs does not require the equatorial–axial–equatorial 1,2,3-trisphosphate motif, and is thus a separate property from their capacity to inhibit the iron-catalysed production of hydroxyl radical. Therefore all-equatorial vicinal trisphosphate groups, as present in Ins(1,3,4,5,6)P 5, appear to support Fe3+ complexation of at least as high affinity as the 1,2,3-trisphosphate motif, but with the difference that iron is not prevented effectively from participating in the Fenton reaction.
Biological predictions for Ins(1,3,4,5,6)P 5 under cytosolic/nuclear conditions of mammalian cells
Although other localizations have not been ruled out, most Ins(1,3,4,5,6)P 5 in mammalian cells is thought to be present in the cytosolic and/or nuclear compartment(s) [26]. In yeast cells at least, the metabolite appears to be able to diffuse freely between cytosol and nucleus; this implies that at least a significant portion of the compound is protein-free and/or forming only low molecular mass complexes [27]. Therefore, our data for the complexation behaviour of Ins(1,3,4,5,6)P 5 in solution, together with the known concentrations of major cations in cytosol/nucleus, can be used for predicting the probable major form(s) of the compound in living cells. Solubility constants are not needed for these calculations because the data presented below indicate that Ins(1,3,4,5,6)P 5 cannot be expected to precipitate at its reported concentration range in mammalian cells of approximately 10–100 μM [28–30] (a different situation arises with certain non-mammalian erythrocytes, as further discussed below). For the calculations, we chose 50 μM Ins(1,3,4,5,6)P 5, 150 mM K+ and pH 7.4 [31]. We further chose concentrations of free Mg2+ that correspond approximately to the extremes and midpoint of the current estimated range (0.25–1 mM) [32]; note that setting the concentration of free Mg2+ to a particular approximate value in this system means setting the total concentration of Mg2+ at values that are slightly higher, so as to allow for the amount of Mg2+ that is complexed by Ins(1,3,4,5,6)P 5. The results (Table 3) predict that Ins(1,3,4,5,6)P 5 is partly associated with Mg2+ but partly unbound to cations. A small proportion is also predicted to be associated with K+. The Mg2+-associated Ins(1,3,4,5,6)P 5 is predicted to consist mostly of the neutral complex [Mg4(H2L)] but also of the anionic 1:1 species [Mg(H3L)]5−. The cation-free Ins(1,3,4,5,6)P 5 is predicted to exist predominantly as the highly charged species [H4L]6− and [H3L]7−. This contrasts with the behaviour of InsP 6, which is predicted to associate fully with Mg2+, as [Mg5(H2L)], under the same conditions [14].
We had previously shown that InsP 6, because of its association with Mg2+, cannot bind Fe3+ under simulated cytosolic/nuclear conditions [14]. In contrast, Ins(1,2,3)P 3 associates much more weakly with Mg2+, and is able to bind fully Fe3+ present in equimolar amounts [16]. The weak association with Mg2+ and the high cellular concentration of Ins(1,3,4,5,6)P 5 mean that if a small concentration of Fe3+ (representing the “chelatable iron pool”, the size of which is unknown [33]) is included in the simulations, this iron associates completely with Ins(1,3,4,5,6)P 5 (Table 3). However, we feel unsure about the biological significance of this result since cellular iron ligands are expected to prevent the participation of iron in the Fenton reaction, a property that Ins(1,3,4,5,6)P 5 lacks.
Ins(1,3,4,5,6)P 5 solids with calcium and magnesium: synthesis and IR spectra
The interaction of M2+ ions with Ins(1,3,4,5,6)P 5 under metal excess gives rise to the formation of fairly insoluble compounds. We prepared and analysed the solids with Ca2+ and Mg2+. The elemental and thermogravimetric analyses agreed with the general formula [M4(H2L)]·xH2O [x = 23 (Mg), 16 (Ca)]. Therefore, the calcium and magnesium solids have the same metal-to-ligand stoichiometry as the neutral tetrametallic complexes formed with these cations in solution. The solids, as expected, also include a number of water molecules, which are lost during thermogravimetric analysis across a wide temperature range, namely between 50 and 210 °C.
Table 4 shows the most intense and characteristic bands present in the IR spectra of (NH4)6H4L, [Mg4(H2L)]·23H2O and [Ca4(H2L)]·16H2O. The ammonium salt has five phosphate groups and four acidic protons. Owing to the extensive proton sharing reported for InsP 6 [34], we can assume that all the phosphate groups are linked to at least one hydrogen. Accordingly, the IR spectrum obtained is similar to that of H2PO4 −, and can be interpreted using this species as a model [35]. The O–H, N–H and C–H bond stretching peaks appear as a wide and intense band in the interval 2,500–3,600 cm−1 [35–37]. A sharp and intense peak at 1,400 cm−1 can be assigned to the H–N–H bending mode of the ammonium cation [37]. Finally, the vibrations involving the phosphate groups appear in the interval 500–1,200 cm−1. The O–P–O bending mode is found at about 514 cm−1, while the symmetric and antisymmetric stretching modes are observed at about 850 and 980 cm−1, respectively [35]. Probably the stretching of the C–O bond also falls in this region [37]. Three more bands of great intensity are observed at 1,058, 1,128 and 1,183 cm−1, and can be assigned to the symmetric and antisymmetric stretching of the PO2 − group [35]. It is possible to analyse the contributions of the phosphate groups to some of the bands by using the IR study on dodecasodium phytate reported by He et al. [38]. Assuming that there are mainly two groups of phosphates in the ligand, those bound to C-1 and C-3 would have an important contribution to the bands at 812 and 932 cm−1, while the bands at 850 and 981 cm−1 could be associated with the vibration of those phosphate groups on C-4, C-5 and C-6. It is worth mentioning that, as expected, the bands mainly related with the vibrations of C-2 phosphate group are not present in the experimental spectrum [38].
The IR spectra of the magnesium and calcium solids are very similar, indicative of isostructural compounds, differing only in the number of water molecules. A similar behaviour was observed for the magnesium and calcium solids of InsP 6 [13]. The δ(H–N–H) peak is absent from the spectra of the magnesium and calcium solids of Ins(1,3,4,5,6)P 5, attesting to a complete NH4 +–Mg2+/Ca2+ exchange during the syntheses. Complexation with the M(II) ions introduces several changes in the IR spectra (in comparison with the spectrum of the ammonium salt), the normal modes associated with phosphate groups being the most affected. The stretching signals of the PO2 – group (five bands in the ligand) appear now as two intense peaks at about 990 and 1,120 cm−1, which are probably associated with ν s(PO2 −) and ν as(PO2 −), respectively. This fact (only two sharp and broad bands, with a small difference between them) was already reported for Ca2+ and Mg2+ solids of InsP 6 [13, 38]. Besides, the frequency for the O–P–O bending mode changes upon coordination to higher wavenumber values. These two facts suggest that the metal cations are bound to the phosphate groups, possibly by means of a direct and bidentate M–O–P coordination [13].
Solubility of Ins(1,3,4,5,6)P 5 solids with calcium and magnesium
The Mg2+ and Ca2+ solids of Ins(1,3,4,5,6)P 5 are only sparingly soluble in water. Therefore, a complete, biologically relevant description of the chemical systems containing Ins(1,3,4,5,6)P 5 and these two cations requires the evaluation of the solubility product constants, K s0 = [M2+]4[H2L8−]. Determination of the K s0 values obviously requires quantification of the concentrations of H2L8− and of the free metal ions at equilibrium with the solids. These can in turn be calculated from the straightforward analytical data by means of appropriate software programs such as HySS [21], fed with the complete set of equilibrium constants for the protonation and complexation equilibria, as reported previously by us for InsP 6 [13]. We thus obtained values for K s0,
which are valid at I = 0.15 M Me4NCl and 37.0 °C. The lower K s0 value for calcium reflects the lower solubility of the Ca2+ solid in comparison with the Mg2+ one.
Figure 7 shows the speciation of Ins(1,3,4,5,6)P 5 in the presence of Ca2+ and of Mg2+, under the conditions which applied for Fig. 4 [1 mM Ins(1,3,4,5,6)P 5, 1 mM M2+], but now including the solubility behaviours (and plotting in terms of total ligand instead of metal). It can be seen that while a small amount of the Ca2+ solid is predicted to form at pH values near neutrality, the more soluble Mg2+ salt does not precipitate under the same conditions. As further discussed below, the prerequisite for significant precipitation to take place in these systems is the dominance in solution of the [M4(H2L)] complexes, and this occurs only under considerable metal excess.
Complete description of the behaviour of Ins(1,3,4,5,6)P 5 in the presence of Ca2+ and/or Mg2+
The protonation constants of Ins(1,3,4,5,6)P 5, together with the Ca2+ and Mg2+ complexation constants and the K s0 values, allow a complete description of the speciation of Ins(1,3,4,5,6)P 5 in the presence of Ca2+/Mg2+. Broadly, the behaviour is characterized by the predominance of soluble 1:1 species under Ins(1,3,4,5,6)P 5 excess and the predominance of the tetrametallic species [M4(H2L)] under metal excess. The [M4(H2L)] complexes exist in solution up to fixed concentration limits, and any amount of them that forms in excess of those limits undergoes precipitation. Such concentration limits are given by the product of the value of each stability constant (4M2+ + H2L8− ↔ [M4(H2L)]) and the corresponding value of K s0 [13], and they thus are 135 μM for [Mg4(H2L)] and 56 μM for [Ca4(H2L)]. Therefore, under conditions of predominance of the [M4(H2L)] complexes [large excess of M2+ with respect to Ins(1,3,4,5,6)P 5, neutral or alkaline pH], these fixed values correspond in practice to the total solubility of Ins(1,3,4,5,6)P 5.
The behaviour of Ins(1,3,4,5,6)P 5 in the presence of Mg2+ or Ca2+ at pH 7.5 is plotted in Fig. 8. For a given cation concentration, increasing the concentration of the ligand causes the abundance of the 1:1 complexes to increase monotonously (Fig. 8a, d). In contrast, the 4:1 species increase and then decrease in abundance, as excess Ins(1,3,4,5,6)P 5 draws the equilibrium towards the 1:1 complexes. The 4:1 complexes accumulate initially as soluble species, but once their solubility limits have been reached (see the “plateaus” in Fig. 8b, e), their additional accumulation takes place with the formation of solids (Fig. 8c, f). On the other hand, if the Ins(1,3,4,5,6)P 5 concentration is held constant and the metal concentration is increased, there is an initial rise in the abundance of 1:1 complexes (Fig. 8a, d), followed by a later increase in the abundance of 4:1 complexes, and finally a decrease in the abundance of 1:1 complexes. The abundance of the 4:1 species in soluble form increases and [as long as the total Ins(1,3,4,5,6)P 5 concentration exceeds 135 μM for Mg2+ and 56 μM for Ca2+] they later precipitate (Fig. 8b, c, e, f; see insets in Fig. 8c, f).
Behaviour of Ins(1,3,4,5,6)P 5 in the presence of magnesium (a–c) and calcium (d–f). The graphs show the predicted abundances of the sum of the different soluble 1:1 complexes [a (Mg 1:1 soluble complexes), d (Ca 1:1 soluble complexes)], of the soluble 4:1 complex [b (Mg 4:1 soluble complex), e (Ca 4:1 soluble complex)], and of the solid tetrametallic complex [c (Mg solid), f (Ca solid)], all plotted against total concentrations of Ins(1,3,4,5,6)P 5 (0.01–10 mM) and M(II) (1–10 mM). In c and f, the insets show “zoom-ins” of the low total Ins(1,3,4,5,6)P 5 range, in which formation of the solids first appears. Predictions are drawn for pH 7.5, and 37.0 °C
The differences between the plots for Mg2+ and Ca2+ are slight, except for the fact that the lower solubility of [Ca4(H2L)] with respect to [Mg4(H2L)] causes the solid to be present across a wider range of conditions in the case of Ca2+. When both Ca2+ and Mg2+ are present, the system behaves in a way similar to what has been described, as long as equal total divalent cation concentrations are considered. Under conditions of total cation excess over Ins(1,3,4,5,6)P 5, precipitation of the more insoluble Ca2+ complex will be favoured over that of the Mg2+ one.
Overall, this behaviour of Ins(1,3,4,5,6)P 5 is similar that of InsP 6 that we reported previously [14] except for the facts that in the InsP 6 system (1) the stoichiometry of the neutral polymetallic InsP 6 complex is 5:1 and (2) the solubility limit of the Mg2+ complex is 49 μM and that of the Ca2+ one is too low to be measured. An additional finer difference between the systems is that the size of the cation excess needed for complete predominance of the neutral polymetallic species over the anionic 1:1 complexes is smaller for InsP 6 than for Ins(1,3,4,5,6)P 5: while systems with InsP 6 and either Mg2+ or Ca2+ at 5:1 metal-to-ligand ratios display [Mg5(H2L)] as practically the sole species, similar systems for Ins(1,3,4,5,6)P 5 (with 4:1 metal-to-ligand ratios) display a mixture of [Mg4(H2L)] and 1:1 species.
Biological predictions for Ins(1,3,4,5,6)P 5 present at high concentrations in non-mammalian erythrocytes
Avian and turtle erythrocytes contain very high (millimolar) concentrations of Ins(1,3,4,5,6)P 5 (reviewed in [11]). Even if the compound has been proposed to interact with (and modulate the oxygen affinity of) haemoglobin, it is unlikely that the whole of the Ins(1,3,4,5,6)P 5 present in these cells is bound to haemoglobin. We ran calculations to predict the physicochemical status of non-haemoglobin-bound Ins(1,3,4,5,6)P 5 in red blood cells, using as conditions 150 mM K+ and pH 7.4. We explored Ins(1,3,4,5,6)P 5 concentrations of 1, 3 and 7 mM: this spans the concentrations reported for avian and turtle erythrocytes [39, 40], and takes into account the possibility that the pool of haemoglobin-free Ins(1,3,4,5,6)P 5 is smaller than the total Ins(1,3,4,5,6)P 5 one. Also, the 7 mM figure in particular covers the extremely high Ins(1,3,4,5,6)P 5 concentrations surprisingly found in the erythrocytes of the Amazonian fish pirarucu during its air-breathing phase (reviewed in [1]). For each Ins(1,3,4,5,6)P 5 concentration we explored different figures for the total concentration of Mg2+, so as to obtain free Mg2+ in the 0.2-mM range reported for (mammalian) erythrocytes [41]. The calculations predicted the whole of Ins(1,3,4,5,6)P 5 to be soluble (as a mixture of non-complexed anion, K+ complexes, and 1:1 Mg2+ complexes, plus a small proportion of [Mg4(H2L)]). Precipitation was predicted to start only at higher free Mg2+ concentrations, i.e. approximately 0.32 mM free Mg2+ for 7 mM Ins(1,3,4,5,6)P 5 and approximately 0.41 mM free Mg2+ for 3 mM Ins(1,3,4,5,6)P 5. Interestingly, InsP 6 was predicted (on the basis of the data in [13]) to be fully precipitated at approximately 0.2 mM free Mg2+, hinting that Ins(1,3,4,5,6)P 5 might have been selected for its function in erythrocytes partly as a consequence of its solubility in the presence of Mg2+.
Biological predictions for Ins(1,3,4,5,6)P 5 under extracellular conditions
It is relevant to predict the speciation of Ins(1,3,4,5,6)P 5 under high-Ca2+, high-Mg2+ conditions such as those prevalent in the extracellular medium of mammals because (1) such conditions correspond to those experiments in which the compound is added to culture cells in physiological media and (2) concentrations of inositol pentakisphosphates in the 10–20-nM range have been reported for rat plasma [42] (although the accompanying measurements for InsP 6 have been questioned [43]). We therefore chose 150 mM Na+, pH 7.5, 2 mM total Ca2+ and 2 mM total Mg2+ to simulate extracellular-like conditions. We first ran a simulation with Ins(1,3,4,5,6)P 5 at 15 nM, i.e. a concentration similar to that reported by Grases et al. [42]: the result shows that plasma Ins(1,3,4,5,6)P 5, if present in the reported concentration range and not bound to proteins, would exist predominantly as a soluble mixture of soluble [Ca4(H2L)] (93%) and [Mg4(H2L)] (6%). We then ran simulations with increasing amounts of Ins(1,3,4,5,6)P 5, so as to determine its maximum solubility in plasma: under the conditions detailed above, 60–65 μM Ins(1,3,4,5,6)P 5 can exist in solution, mostly as the mixture [Mg4(H2L)]/[Ca4(H2L)]. The solubility in intracellular vesicular compartments, similarly rich in Ca2+ and Mg2+ as the extracellular medium but more acidic, will be in excess of the value given above. Therefore, the physicochemical properties of Ins(1,3,4,5,6)P 5 would allow the existence of a significant pool of soluble, protein-free compound in the extracellular medium as well as in intracellular vesicular compartments.
Practical data for the experimentation with Ins(1,3,4,5,6)P 5
Our data provide a few simple guidelines to keep experiments using added Ins(1,3,4,5,6)P 5 within reasonably physiological conditions. When mimicking cytosolic/nuclear conditions, one must reason that Ins(1,3,4,5,6)P 5 can complex up to 4 mol of Mg2+ per mole, although under most conditions except for very large Mg2+ excesses and/or very high pH, this will be less than 1 mol per mole (see, e.g., Table 3). Since cytosol and nucleus of mammalian cells contain 0.25–1 mM free Mg2+ [32], the total concentration of Mg2+ included in the experiments must be in excess of the molar concentration of Ins(1,3,4,5,6)P 5 (plus the Mg2+-complexating capacity of any other chelators such as ATP that are present). Under these conditions Ins(1,3,4,5,6)P 5 will remain in solution up to 135 μM (or higher, but this only for a very restricted subset of conditions).
For procedures involving other conditions (e.g. the preparation of stock solutions, experiments mimicking intestinal conditions, assays of enzymatic activities with biotechnological purposes), a very important practical point is to know in advance whether Ins(1,3,4,5,6)P 5 will be soluble or will precipitate. The solubility of Ins(1,3,4,5,6)P 5 is determined by multiple linked equilibria (solid formation as such plus the various complexation and protonation equilibria in solution), and hence the constants reported in this paper can only be put into practice with the help of a specialized software program such as HySS. We have thus put together a series of plots that summarize the solubility behaviour of Ins(1,3,4,5,6)P 5 in the presence of Ca2+ and Mg2+ (Fig. 9). In these plots, the frontiers between solubility and precipitation are given in terms of total concentrations of Ins(1,3,4,5,6)P 5 and metal. Overall, for each given condition, the area of dominance of the solids is wedged in the plots within the region of full solubility, which encompasses both low and high Ins(1,3,4,5,6)P 5 concentrations. As mentioned before, the subregions of low and high Ins(1,3,4,5,6)P 5 concentration are dominated by 4:1 and the 1:1 complexes, respectively. The “wedges” of precipitation in the plots have mostly “horizontal” “lower boundaries”, which correspond to the fixed values for the solubility of the [M4(H2L)] complexes mentioned above. However, these “horizontal boundaries” do not apply in the low metal concentration range (below 5 mM M2+ for pH 7.5; below 20 mM Ca2+ or 60 mM Mg2+ for pH 5.0), in which the 1:1 complexes become increasingly significant and add to the total solubility of Ins(1,3,4,5,6)P 5. In fact, going towards low M2+ concentrations, one reaches a value below which Ins(1,3,4,5,6)P 5, irrespective of its concentration, does not precipitate as Ca2+ or Mg2+ salt; these values are 0.65 mM for Ca2+ and 1.37 mM for Mg2+ at pH 7.5, and increase substantially as the pH is lowered. It must be borne in mind that while the plots in Fig. 9 are given in terms of total metal ion, for some purposes what is known and/or fixed is the concentration of free metal ion, as is the case with in vivo experiments.
The solubility behaviour of Ins(1,3,4,5,6)P 5, as predicted by the equilibrium equations, in the presence of Ca2+ [a (Ca, pH 7.5), b (Ca, pH 5.0)] or Mg2+ [c (Mg, pH 7.5), d (Mg, pH 5.0)] plotted for pH 7.5 and 5.0. The “frontier lines” drawn correspond to conditions in which either 1% or 99% of Ins(1,3,4,5,6)P 5 present is predicted to exist as a solid
References
Irvine RF, Schell MJ (2001) Nat Rev Mol Cell Biol 2:327–338
Johnson LF, Tate ME (1969) Can J Chem 47:63–73
Stephens LR, Hawkins PT, Stanley AF, Moore T, Poyner DR, Morris PJ, Hanley MR, Kay RR, Irvine RF (1991) Biochem J 275:485–499
Guse AH, Emmrich F (1991) J Biol Chem 266:24498–24502
McConnell FM, Stephens LR, Shears SB (1991) Biochem J 280:323–329
Verbsky JW, Wilson MP, Kisseleva MV, Majerus PW, Wente SR (2002) J Biol Chem 277:31857–31862
Shears SB (2007) Biochem Soc Symp 74:211–221
Frederick JP, Mattiske D, Wofford JA, Megosh LC, Drake LY, Chiou ST, Hogan BL, York JD (2005) Proc Natl Acad Sci USA 102:8454–8459
Komander D, Fairservice A, Deak M, Kular GS, Prescott AR, Peter Downes C, Safrany ST, Alessi DR, van Aalten DM (2004) EMBO J 23:3918–3928
Jackson SG, Zhang Y, Haslam RJ, Junop MS (2007) BMC Struct Biol 7:80–90
Gao Y, Wang HY (2007) J Biol Chem 282:26490–26502
Shears SB (2001) Cell Signal 13:151–158
Veiga N, Torres J, Domínguez S, Mederos A, Irvine RF, Díaz A, Kremer C (2006) J Inorg Biochem 100:1800–1810
Torres J, Domínguez S, Cerdá MF, Obal G, Mederos A, Irvine RF, Díaz A, Kremer C (2005) J Inorg Biochem 99:828–840
Torres J, Veiga N, Gancheff J, Domínguez S, Mederos A, Sundberg M, Sánchez A, Castiglioni J, Díaz A, Kremer C (2008) J Mol Struct 874:77–88
Veiga N, Torres J, Mansell D, Freeman S, Domínguez S, Barker CJ, Díaz A, Kremer C (2009) J Biol Inorg Chem 14:51–59
Schwarzenwach G, Flaschka H (1969) Complexometric titrations, 2nd edn. Methuen, London
Godage HY, Riley AM, Woodman TJ, Potter BVL (2006) Chem Commun 2989–2991
Gans P, O’Sullivan B (2000) Talanta 51:33–37
Gans P, Sabatini A, Vacca A (1996) Talanta 43:1739–1753
Alderighi L, Gans P, Ienco A, Peters D, Sabatini A, Vacca A (1999) Coord Chem Rev 184:311–318
Riley AM, Trusselle M, Kuad P, Borkovec M, Cho J, Choi JH, Qian X, Shears SB, Spiess B, Potter BVL (2006) Chembiochem 7:1114–1122
Mansell D, Rattray N, Etchells L, Schwalbe CH, Blake AJ, Bichenkova EV, Bryce R, Barjer CJ, Díaz A, Kremer C, Freeman S (2008) Chem Commun 5161–5163
Hawkins PT, Poyner DR, Jackson TR, Letcher AJ, Lander DA, Irvine RF (1993) Biochem J 294:929–934
Spiers ID, Barker CJ, Chung S-K, Chang Y-T, Freeman S, Gardiner JM, Hirst PH, Lambert PA, Michell RH, Poyner DR, Schwalbe CH, Smitn AW, Solomons KRH (1996) Carbohydr Res 282:81–99
Stuart JA, Anderson KL, French PJ, Kirk CJ, Michell RH (1994) Biochem J 303:517–525
Miller AL, Suntharalingam M, Johnson SL, Audhya A, Emr SD, Wente SR (2004) J Biol Chem 279:51022–51032
French PJ, Bunce CM, Stephens LR, Lord JM, McConell FM, Brown G, Creba JA, Michell RH (1991) Proc R Soc Lond B 245:193–201
Bunce CM, French PJ, Allen P, Mountford JC, Moor B, Greaves MF, Michell RH, Brown G (1993) Biochem J 289:667–673
Barker CJ, Wright J, Hughes PJ, Kirk CJ, Michell RH (2004) Biochem J 380:465–473
Wu MM, Llopis J, Adams JM, McCaffery MS, Kulomaa TE, Machen TE, Moore HP, Tsien RY (2000) Chem Biol 7:197–209
Grubbs RD (2002) Biometals 15:251–259
Kakhlon O, Kabantch I (2002) Free Radic Biol Med 33:1037–1046
Isbrandt LR, Oertel RP (1980) J Am Chem Soc 102:3144–3148
Chapman AC, Thirlwell LE (1964) Spectrochim Acta 20:937–947
Rajkumar BJM, Ramakrishnan V (2001) Spectrochim Acta A 57:247–254
Lin-Vien D, Colthup NB, Fateley WG, Graselli JG (1991) The handbook of infrared and Raman characteristic frequencies of organic molecules. Academic Press, San Diego
He Z, Honeycutt CW, Zhang T, Bertsch PM (2006) J Environ Qual 35:1319–1328
Bartlett GR (1976) Comp Biochem Physiol A 55:211–214
Villar JL, Puigbò P, Riera-Codina M (2003) Comp Biochem Physiol B 135:169–175
Günther T (2007) Magnes Res 20:161–167
Grases F, Simonet BM, Prieto RM, March JG (2001) J Nutr Biochem 12:595–601
Letcher AJ, Schell MJ, Irvine RF (2008) Biochem J 416:263–270
Acknowledgments
N.V. is indebted to PEDECIBA-Química and ANII for a scholarship. We acknowledge support from the Wellcome Trust (programme grant 082837 to A.M.R. and B.V.L.P.). Thermal analyses were carried out by Jorge Castiglioni, LAFIDESU, DETEMA, Facultad de Química (Uruguay). A.D. is grateful to the Biochemical Society for a bursary to attend the Harden conference on inositol phosphates and lipids.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Veiga, N., Torres, J., Godage, H.Y. et al. The behaviour of inositol 1,3,4,5,6-pentakisphosphate in the presence of the major biological metal cations. J Biol Inorg Chem 14, 1001–1013 (2009). https://doi.org/10.1007/s00775-009-0510-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-009-0510-z