Abstract
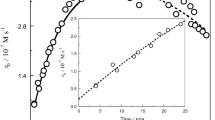
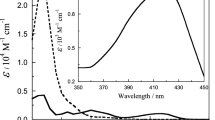
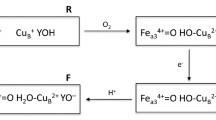
The catalase from Proteus mirabilis peroxide-resistant bacteria is one of the most efficient heme-containing catalases. It forms a relatively stable compound II. We were able to prepare samples of compound II from P. mirabilis catalase enriched in 57Fe and to study them by spectroscopic methods. Two different forms of compound II, namely, low-pH compound II (LpH II) and high-pH compound II (HpH II), have been characterized by Mössbauer, extended X-ray absorption fine structure (EXAFS) and UV-vis absorption spectroscopies. The proportions of the two forms are pH-dependent and the pH conversion between HpH II and LpH II is irreversible. Considering (1) the Mössbauer parameters evaluated for four related models by density functional theory methods, (2) the existence of two different Fe–Oferryl bond lengths (1.80 and 1.66 Å) compatible with our EXAFS data and (3) the pH dependence of the α band to β band intensity ratio in the absorption spectra, we attribute the LpH II compound to a protonated ferryl FeIV–OH complex (Fe–O approximately 1.80 Å), whereas the HpH II compound corresponds to the classic ferryl FeIV=O complex (Fe=O approximately 1.66 Å). The large quadrupole splitting value of LpH II (measured 2.29 mm s−1 vs. computed 2.15 mm s−1) compared with that of HpH II (measured 1.47 mm s−1 vs. computed 1.46 mm s−1) reflects the protonation of the ferryl group. The relevancy and involvement of such (FeIV=O/FeIV–OH) species in the reactivity of catalase, peroxidase and chloroperoxidase are discussed.








Similar content being viewed by others
Abbreviations
- CPO:
-
Chloroperoxidase
- DFT:
-
Density functional theory
- DW:
-
Debye–Waller
- EPR:
-
Electron paramagnetic resonance
- EXAFS:
-
Extended X-ray absorption fine structure
- HpH II:
-
High-pH Proteus mirabilis catalase compound II
- HRP:
-
Horseradish peroxidase
- LpH II:
-
Low-pH Proteus mirabilis catalase compound II
- MLC:
-
Micrococcus lysodeikticus catalase
- MO:
-
Molecular orbital
- PMC:
-
Proteus mirabilis catalase
- TMP:
-
Tetramesitylporphyrin
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Hauptmann N, Cadenas E (1997) In: Scandalios JG (eds) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, New York, pp 1–20
Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS (2005) Science 308:1909–1911
Jang BC, Paik JH, Kim SP, Shin DH, Song DK, Park JG, Suh MH, Park JW, Suh SI (2005) Cell Signal 17:625–633
Nicholls P, Fita I, Loewen PC (2001) Adv Inorg Biochem 51:51–106
Lardinois OM, Mestdagh MM, Rouxhet PG (1996) Biochim Biophys Acta 1295:222–238
Lardinois OM (1995) Free Radical Res 22:251–274
Kirkman HN, Rolfo M, Ferraris AM, Gaetani GF (1999) J Biol Chem 274:13908–13914
Andreoletti P, Gambarelli S, Sainz G, Stojanoff V, White C, Desfonds G, Gagnon J, Gaillard J, Jouve HM (2001) Biochemistry 40:13734–13743
Jones P (2001) J Biol Chem 276:13791–13796
Conradie J, Swarts JC, Ghosh A (2004) J Phys Chem B 108:452–456
Conradie J, Wasbotten I, Ghosh A (2006) J Inorg Biochem 100:502–506
Rovira C, Fita I (2003) J Phys Chem B 107:5300–5305
Rovira C (2005) ChemPhysChem 6:1820–1826
Green MT, Dawson JH, Gray HB (2004) Science 304:1653–1656
Hersleth HP, Ryde U, Rydberg P, Gorbitz CH, Andersson KK (2006) J Inorg Biochem 100:460–476
Rydberg P, Sigfridsson E, Ryde U (2004) J Biol Inorg Chem 9:203–223
Silaghi-Dumitrescu R (2004) J Biol Inorg Chem 9:471–476
Green MT (2006) J Am Chem Soc 128:1902–1906
Behan RK, Green MT (2006) J Inorg Biochem 100:448–459
Terner J, Palaniappan V, Gold A, Weiss R, Fitzgerald MM, Sullivan AM, Hosten CM (2006) J Inorg Biochem 100:480–501
Switala J, Loewen PC (2002) Arch Biochem Biophys 401:145–154
Jouve HM, Beaumont F, Léger I, Foray J, Pelmont J (1989) Biochem Cell Biol 67:271–277
Gouet P, Jouve HM, Dideberg O (1995) J Mol Biol 249:933–954
Gouet P, Jouve HM, Williams PA, Andersson I, Andreoletti P, Nussaume L, Hajdu J (1996) Nat Struct Biol 3:951–956
Andreoletti P, Pernoud A, Sainz G, Gouet P, Jouve HM (2003) Acta Crystallogr D Biol Crystallogr 59:2163–2168
Horner O, Oddou J-L, Mouesca J-M, Jouve HM (2006) J Inorg Biochem 100:477–479
Stone KL, Hoffart LM, Behan RK, Krebs C, Green MT (2006) J Am Chem Soc 128:6147–6153
Sauret G, Jouve H, Pelmont J (1979) Can J Microbiol 25:312–320
Jouve H, Sauret G, Laboure AM, Pelmont J (1979) Can J Microbiol 25:302–311
Andreoletti P, Sainz G, Jaquinod M, Gagnon J, Jouve HM (2003) Proteins 50:261–271
Rieske JS, Lipton SH, Baum H, Silman HI (1967) J Biol Chem 242:4888–4896
Ivancich A, Jouve HM, Sartor B, Gaillard J (1997) Biochemistry 36:9356–9364
Jeandey Ch, Horner O, Oddou J-L, Jeandey C (2003) Meas Sci Technol 14:629–632
Horner O, Mouesca JM, Oddou JL, Jeandey C, Niviere V, Mattioli TA, Mathe C, Fontecave M, Maldivi P, Bonville P, Halfen JA, Latour JM (2004) Biochemistry 43:8815–8825
Filipponi A, Di Cicco A (2000) Task Q 4:575–669
Murshudov GN, Grebenko AI, Brannigan JA, Antson AA, Barynin VV, Dodson GG, Dauter Z, Wilson KS, Melik-Adamyan WR (2002) Acta Crystallogr D Biol Crystallogr 58:1972–1982
Filipponi A, Di Cicco A, Natoli CR (1995) Phys Rev B Condens Matter 52:15122–15134
Filipponi A, Di Cicco A (1995) Phys Rev B Condens Matter 52:15135–15149
Borghi E, Solari PL (2005) J Synchrotron Radiat 12:102–110
Borghi E, Solari PL, Beltramini M, Bubacco L, Di Muro P, Salvato B (2002) Biophys J 82:3254–3268
Baerends EJ, Ellis DE, Ros P (1973) Chem Phys 2:41–45
Baerends EJ, Ros P (1973) Chem Phys 2:52–59
Baerends EJ, Ros P (1978) Int J Quantum Chem Quantum Chem Symp 12:169–190
Bickelhaupt FM, Baerends EJ, Ravenek W (1990) Inorg Chem 29:350–354
TeVelde G, Baerends EJ (1992) J Comput Phys 99:84–98
Ziegler T (1991) Chem Rev 91:651–667
Vosko SH, Wilk L, Nusair M (1980) Can J Phys 58:1200
Painter GS (1981) Phys Rev B 24:4264–4270
Becke AD (1988) Phys Rev A 38:3098–3100
Perdew JP (1986) Phys Rev B 33:8822–8824
Groves JT, Quinn RQ, Mc Murry TJ, Nakamura M, Lang G, Boso B (1985) J Am Chem Soc 107:354–360
Zimmermann R, Ritter G, Spiering H, Nagy D (1974) J Phys C 6:439–442
Sitter AJ, Reczek CM, Terner J (1985) J Biol Chem 260:7515–7522
Chuang WJ, Heldt J, Van Wart HE (1989) J Biol Chem 264:14209–14215
Schulz CE, Devaney PW, Winkler H, Debrunner PG, Doan N, Chiang R, Rutter R, Hager LP (1979) FEBS Lett 103:102–105
Schulz CE, Rutter R, Sage JT, Debrunner PG, Hager LP (1984) Biochemistry 23:4743–4754
Oosterhuis WT, Lang G (1973) J Chem Phys 58:4757–4765
Münck E (2000) In: Que LJr (ed) Physical methods in bioinorganic chemistry—spectroscopy and magnetism. University Science Books, chap 6
Rutter R, Hager LP, Dhonau H, Hendrich M, Valentine M, Debrunner P (1984) Biochemistry 23:6809–6816
Leising RA, Brennan BA, Que L Jr, Fox BG, Münck E (1991) J Am Chem Soc 113:3988–3990
Rohde JU, In JH, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Munck E, Nam W, Que L Jr (2003) Science 299:1037–1039
Egawa T, Proshlyakov DA, Miki H, Makino R, Ogura T, Kitagawa T, Ishimura Y (2001) J Biol Inorg Chem 6:46–54
Chang CS, Yamazaki I, Sinclair R, Khalid S, Powers L (1993) Biochemistry 32:923–928
Dunford HB (1999) Heme peroxidases. Wiley, New York
Rosa A, Ricciardi G, Baerends EJ, van Gisbergen SJA (2001) J Phys Chem A 105:3311–3327
Gouterman M (1978) In: Dolphin D (ed) The porphyrins, vol 3. Academic, New York, pp 1–165
Penner-Hahn JE, Eble KS, McMurry TJ, Renner M, Balch AL, Groves JT, Dawson JH, Hodgson KO (1986) J Am Chem Soc 108:7819–7825
Chance M, Powers L, Kumar C, Chance B (1986) Biochemistry 25:1259–1265
Chance M, Powers L, Poulos T, Chance B (1986) Biochemistry 25:1266–1270
Stern EA (2001) J Synchrotron Radiat 8:49–54
Sastri CV, Park MJ, Ohta T, Jackson TA, Stubna A, Seo MS, Lee J, Kim J, Kitagawa T, Munck E, Que L Jr, Nam W (2005) J Am Chem Soc 127:12494–12495
Bukowski MR, Koehntop KD, Stubna A, Bominaar EL, Halfen JA, Munck E, Nam W, Que L Jr (2005) Science 310:1000–1002
Lang G, Spartalian K, Yonetani T (1976) Biochim Biophys Acta 451:250–258
Schulz CE, Chiang R, Debrunner PG (1979) J Phys 40:C2 534–C2 536
Hashimoto S, Tatsuno Y, Kitagawa T (1986) Proc Natl Acad Sci USA 83:2417–2421
Ivancich A, Mattioli TA, Un S (1999) J Am Chem Soc 121:5743–5753
Proshlyakov DA, Ogura T, Shinzawa-Itoh K, Yoshikawa S, Kitagawa T (1996) Biochemistry 35:8580–8586
Jouve HM, Tessier S, Pelmont J (1983) Can J Biochem Cell Biol 61:8–14
Acknowledgements
N. Genand-Riondet (CEA/Saclay) is gratefully acknowledged for performing the high-field Mössbauer spectroscopy experiments and the European Synchrotron Radiation Facility is gratefully acknowledged for provision of synchrotron radiation. Tony Mattioli (CEA/Saclay) is thanked for resonance Raman measurements. We would like to thank Catherine Bougault (IBS, Grenoble) for helpful discussions and Elizabeth Hewat (IBS, Grenoble) for corrections of the English.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material
: Reference UV-visible spectra of PMC resting state, compound I and compound II (Fig. S1); Mössbauer spectra of as-isolated 57Fe catalase from P. mirabilis at 4.2 K in a magnetic field of 50 mT and 7.0 T applied parallel to the γ-beam (Fig. S2); Mössbauer spectrum of compound I in 57Fe catalase from P. mirabilis at 40 K in a magnetic field of 3T applied parallel to the γ-beam (Fig. S3); Mössbauer spectrum of compound II in 57Fe catalase from P. mirabilis at 150 K in a magnetic field of 7.0 T applied parallel to the γ-beam (Fig. S4);comparison between the visible absorption spectra of compound II samples used for EXAFS and Mössbauer measurements (Fig. S5); experimental EXAFS of PMC compound II from P. mirabilis at pH 8.0 with results of the EXAFS analysis considering two distinct iron–oxo contributions (Fig. S6); projection of the minimization function on the (R–Fe=O, R–Fe–OH) plane, i.e. contour plot (regions enclosed by squares correspond to the 95% confidence interval) (Fig. S7); linear correlation between the computed quadrupole splitting ΔEQ and the computed electronic density at the iron nucleus ρ(Fe), by using all six FeIV=O models of compound II at high pH and four possible FeIV–OH models of compound II at low pH (Figure S8); optimized coordinates for the models 1, 1ter, 2, 2ter, 3, 3bis and 4 (Table S1 a–g); structural parameters and quadrupole splitting in case of the alternative protonation of the axial Tyrosine residue (here without cation) (Table S2); repartition of the iron spin population (%) among the d atomic orbitals for the models 1 to 4. Summation per spin (∑dαβ) and total iron spin populations (∑dα−∑dβ) (Table S3); mononuclear iron biomolecules and complexes used for establishing the linear correlation between experimentally measured isomer shifts at 4.2 K and experimentally measured quadrupole splitting at 4.2 K (Table S4). (DOC 687 kb)
Rights and permissions
About this article
Cite this article
Horner, O., Mouesca, JM., Solari, P.L. et al. Spectroscopic description of an unusual protonated ferryl species in the catalase from Proteus mirabilis and density functional theory calculations on related models. Consequences for the ferryl protonation state in catalase, peroxidase and chloroperoxidase. J Biol Inorg Chem 12, 509–525 (2007). https://doi.org/10.1007/s00775-006-0203-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-006-0203-9