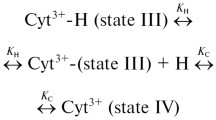The structural and redox properties of a non-covalent complex reconstituted upon mixing two non-contiguous fragments of horse cytochrome c, the residues 1–38 heme-containing N-fragment with the residues 57–104 C-fragment, have been investigated. With respect to native cyt c, the complex lacks a segment of 18 residues, corresponding, in the native protein, to an omega (Ω)-loop region. The fragment complex shows compact structure, native-like α-helix content but a less rigid atomic packing and reduced stability with respect to the native protein. Structural heterogeneity is observed at pH 7.0, involving formation of an axially misligated low-spin species and consequent partial displacement of Met80 from the sixth coordination position of the heme-iron. Spectroscopic data suggest that a lysine (located in the Met80-containing loop, namely Lys72, Lys73, or Lys79) replaces the methionine residue. The residues 1–38/57–104 fragment complex shows an unusual biphasic alkaline titration characterized by a low (pKa1=6.72) and a high pKa-associated state transition (pKa2=8.56); this behavior differs from that of native cyt c, which shows a monophasic alkaline transition (pKa=8.9). The data indicate that the 40s Ω-loop plays an important role in the stability of cyt c and in ensuring a correct alkaline conformational transition of the protein.








Similar content being viewed by others
Abbreviations
- CD:
-
circular dichroism
- CV:
-
cyclic voltammetry
- cyt c:
-
cytochrome c
- RR:
-
resonance Raman
References
Kuwajima K (1989) Proteins 6:87–103
Creighton TE (1990) Biochem J 270:1–15
Kim PS, Baldwin RL (1990) Annu Rev Biochem 59:631–660
Ptitsyn OB (1992) In: Creighton TE (ed) Protein folding. Freeman, New York, pp 243–300
Privalov PL (1996) J Mol Biol 258:707–725
Fontana A, Zambonin M, De Filippis V, Bosco M, Polverino de Laureto P (1995) FEBS Lett 362:266–270
Moore GR, Pettigrew GW (1990) Cytochromes c. Evolutionary, structural and physiological aspects. Springer, Berlin Heidelberg New York
Sosnick TR, Mayne L, Hiller R, Englander SW (1994) Nat Struct Biol 1:149–156
Colón W, Roder H (1996) Nat Struct Biol 3:1019–1025
Takahashi S, Yeh S-R, Das TK, Chan C-K, Gottfried DS, Rousseau DL (1997) Nat Struct Biol 4:44–50
Yeh, S-R, Takahashi S, Fan B, Rousseau DL (1997) Nat Struct Biol 4:51–56
Bai Y (1999) Proc Natl Acad Sci USA 96:477–480
Pierce MM, Nall BT (2000) J Mol Biol 298:955–969
Jeng MF, Englander SW (1991) J Mol Biol 221:1045–1061
Russell BS, Melenkivitz R, Bren K (2000) Proc Natl Acad Sci USA 97:8312–8317
Jordan T, Eads JC, Spiro TG (1995) Protein Sci 4:716–728
Oellerich S, Wackerbarth H, Hildebrandt P (2002) J Phys Chem B 106:6566–6580
Sinibaldi F, Howes BD, Smulevich G, Ciaccio C, Coletta M, Santucci R (2003) J Biol Inorg Chem 8:663–670
Goto Y, Takahashi N, Fink AL (1990) Biochemistry 29:3480–3488
Marmorino JL, Pielak GJ (1995) Biochemistry 34:3140–3143
Santucci R, Bongiovanni C, Mei G, Ferri T, Polizio F, Desideri A (2000) Biochemistry 39:12632–12638
Wilgus H, Ranweiler JS, Wilson GS, Stellwagen E (1978) J Biol Chem 253:3265–3272
Wallace CJ (1987) J Biol Chem 262:16767–16770
Fisher A, Taniuchi H (1992) Arch Biochem Biophys 296:1–16
Kang X, Carey J (1999) J Mol Biol 285:463–468
Yokota A, Takenaka H, Oh T, Noda Y, Segawa S-I (1998) Protein Sci 7:1717–1727
Santucci R, Fiorucci L, Sinibaldi F, Polizio F, Desideri A, Ascoli F (2000) Arch Biochem Biophys 379:331–336
Santoni E, Scatragli S, Sinibaldi F, Fiorucci L, Santucci R, Smulevich G (2004) J Inorg Biochem 98:1067–1077
Sinibaldi F, Fiorucci L, Mei G, Ferri T, Desideri A, Ascoli F, Santucci R (2001) Eur J Biochem 268:4537–4543
Bushnell GW, Louie GV, Brayer GD (1990) J Mol Biol 214:585–595
Leszcynski JF, Rose GD (1986) Science 234:849–855
Pettigrew GW, Moore GR (1987) Cytochromes c. Biological aspects. Springer, Berlin Heidelberg New York
Jemmerson R, Liu J, Hausauer D, Lam K-P, Mondino A, Nelson RD (1999) Biochemistry 38:3599–3609
Sinibaldi F, Piro MC, Howes BD, Smulevich G, Ascoli F, Santucci R (2003) Biochemistry 42:7604–7610
Hoang L, Maity H, Krishna MMG, Lin Y, Englander SW (2003) J Mol Biol 331:37–43
Korszun ZN, Salemme FR (1977) Proc Natl Acad Sci USA 74:5244–5247
Eddowes MJ, Hill HAO (1979) J Am Chem Soc 101:4461–44641
Berghuis AM, Brayer GD (1992) J Mol Biol 223:959–976
Hoard JL (1973) Ann NY Acad Sci 206:18–31
Spaulding LD, Chang CC, Yu NT, Felton RH (1975) J Am Chem Soc 97:2517–2525
Choi S, Spiro TG, Langry KC, Smith KM, Budd DL, La Mar GN (1982) J Am Chem Soc 104:4345–4351
Sparks LD, Anderson KK, Medforth CJ, Smith K, Shelnutt JA (1994) Inorg Chem 33:2297–2302
Indiani C, De Sanctis G, Neri F, Santos H, Smulevich G, Coletta M (2000) Biochemistry 39: 8234–8242
Dopner S, Hildebrandt P, Rosell FI, Mauk AG (1998) J Am Chem Soc 120:11246–11255
Hu S, Morris IK, Singh JP, Smith KM, Spiro TG (1993) J Am Chem Soc 115:12446–12458
Smulevich G, Bjerrum MJ, Gray HB, Spiro TG (1994) Inorg Chem 33:4629–4634
Zheng J, Ye S, Lu T, Cotton TM, Chumanov G (2000) Biopolymers (Biospectroscopy) 57:77–84
Clark WM (1972) Oxidation-reduction potentials of organic systems. Krieger, Huntington, NY, USA
Santucci R, Reinhard H, Brunori M (1988) J Am Chem Soc 110:8536–8537
Raphael AL, Gray HB (1989) Proteins 6:338–340
Raphael AL, Gray HB (1991) J Am Chem Soc 113:1038–1040
Pielak GJ, Oikawa K, Mauk AG, Smith M, Kay CM (1986) J Am Chem Soc 108:2724–2727
Ascoli F, Santucci R (1996) J Inorg Biochem 68:211–214
Wilson MT, Greenwood C (1996) In: Scott RA, Mauk AG (eds), Cytochrome c. A multidisciplinary approach. University Science Books, Sausalito, Calif., USA, pp 611–634
Stellwagen E, Cass R (1974) Biochem Biophys Res Commun 60:371–375
Pace CN (1975) CRC Crit Rev Biochem: 1–43
Myers JK, Pace CN, Scholtz JM (1995) Protein Sci 4:2138–2148
Ferri T, Poscia A, Ascoli F, Santucci R (1996) Biochim Biophys Acta 1298:102–108
Nelson CJ, Bowler BE (2000) Biochemistry 39:13584–13594
Neri F, Indiani C, Welinder KG, Smulevich G (1998) Eur J Biochem 251:830–838
Wallace CJ, Proudfoot AE (1987) Biochem J 245:773–779
Guex N, Peitsch MC (1997) Electrophoresis 18:2714–2723
Acknowledgements
The authors wish to thank P. Sarti and E. Forte (Dipartimento di Biochimica, Università di Roma “La Sapienza”) for assistance in the polarographic measurements, and E. Schininà (Dipartimento di Biochimica, Università di Roma “La Sapienza”) for mass spectrometric measurements. This research was funded in part by grants from the Italian MIUR (COFIN 2001 031798).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caroppi, P., Sinibaldi, F., Santoni, E. et al. The 40s Ω-loop plays a critical role in the stability and the alkaline conformational transition of cytochrome c. J Biol Inorg Chem 9, 997–1006 (2004). https://doi.org/10.1007/s00775-004-0601-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-004-0601-9