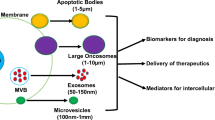
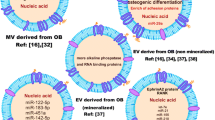
Abstract
Extracellular vesicles (EVs) are small particles with lipid bilayer membranes that are secreted by all cell types and are widely known as crucial intercellular communication mediators, shuttling biologically active molecules. The bone is a typically preferred site of cancer metastasis due to its unique cellular compositions and dynamics. Bone cell-derived EVs serve as regulators that orchestrate harmonious bone homeostasis. Cancer cells secrete specific EVs in a series of the bone metastatic process to dominate the bone microenvironment. Additionally, cancer cell-related EVs contribute to pre-metastatic niche formation, bone homeostasis disruption, and tumor bone progression and survival. Here, we investigated recent studies on EV-mediated crosstalk in the bone tumor microenvironment. Furthermore, this review aimed to elucidate the EV-based therapeutic perspectives for bone metastasis.


Similar content being viewed by others
References
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133:571–573. https://doi.org/10.1016/S0140-6736(00)49915-0
Whiteside TL (2008) The tumor microenvironment and its role in promoting tumor growth. Oncogene 27:5904–5912. https://doi.org/10.1038/onc.2008.271
György B, Szabó TG, Pásztói M, Pál Z, Misják P et al (2011) Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68:2667–2688. https://doi.org/10.1007/s00018-011-0689-3
Raposo G, Nijman HW, Stoorvogel W, Leijendekker R, Harding CV et al (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183:1161–1172. https://doi.org/10.1084/jem.183.3.1161
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ et al (2007) Exosome-mediated transfer of MRNAs and MicroRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659. https://doi.org/10.1038/ncb1596
Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y et al (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285:17442–17452. https://doi.org/10.1074/jbc.M110.107821
Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, Van Eijndhoven MAJ, Hopmans ES et al (2010) Functional delivery of viral MiRNAs via exosomes. Proc Natl Acad Sci USA 107:6328–6333. https://doi.org/10.1073/pnas.0914843107
Zhang Y, Liu D, Chen X, Li J, Li L et al (2010) Secreted monocytic MiR-150 enhances targeted endothelial cell migration. Mol Cell 39:133–144. https://doi.org/10.1016/j.molcel.2010.06.010
Yoneda T, Hiraga T (2005) Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun 328:679–687. https://doi.org/10.1016/j.bbrc.2004.11.070
Coleman RE (1997) Skeletal complications of malignancy. Cancer 80:1588–1594. https://doi.org/10.1002/(sici)1097-0142(19971015)80:8+%3c1588::aid-cncr9%3e3.0.co;2-g
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. https://doi.org/10.1083/jcb.201211138
Witwer KW, Théry C (2019) Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles 8:1. https://doi.org/10.1080/20013078.2019.1648167
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD et al (2018) Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. https://doi.org/10.1080/20013078.2018.1535750
Kowal J, Arras G, Colombo M, Jouve M, Morath JP et al (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 113(8):E968–77. https://doi.org/10.1073/pnas.1521230113
Willms E, Cabañas C, Mäger I, Wood MJA, Vader P (2018) Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol 9:738. https://doi.org/10.3389/fimmu.2018.00738
Li P, Kaslan M, Lee SH, Yao J, Gao Z (2017) Progress in exosome isolation techniques. Theranostics 7:789–804. https://doi.org/10.7150/thno.18133
Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M et al (2013) Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies microvesicles and exosomes. J Extracell Vesicles 12:2. https://doi.org/10.3402/jev.v2i0.20677
Andreu Z, Yáñez-Mó M (2014) Tetraspanins in extracellular vesicle formation and function. Front Immunol 5:1–12. https://doi.org/10.3389/fimmu.2014.00442
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3:S131. https://doi.org/10.2215/CJN.04151206
Quarles LD (2008) Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118:3820–3828. https://doi.org/10.1172/JCI36479
Wilson A, Trumpp A (2006) Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6:93–106. https://doi.org/10.1038/nri1779
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342. https://doi.org/10.1038/nature01658
Salhotra A, Shah HN, Levi B, Longaker MT (2020) Mechanisms of bone development and repair. Nat Rev Mol Cell Biol 21:696–711. https://doi.org/10.1038/s41580-020-00279-w
Hughes DE, Salter DM, Dedhar S, Simpson R (1993) Integrin expression in human bone. J Bone Miner Res 8:527–533. https://doi.org/10.1002/JBMR.5650080503
Khosla S (2001) Minireview: The OPG/RANKL/RANK system. Endocrinology 142:5050–5055. https://doi.org/10.1210/endo.142.12.8536
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473:139–146. https://doi.org/10.1016/J.ABB.2008.03.018
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M et al (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231–1234. https://doi.org/10.1038/nm.2452
Sigl V, Jones LP, Penninger JM (2016) RANKL/RANK: from bone loss to the prevention of breast cancer. Open Biol 6(11):160230. https://doi.org/10.1098/rsob.160230
Boyce BF, Xing L (2006) Osteoclasts no longer osteoblast slaves. Nat Med 12:1356–1358. https://doi.org/10.1038/nm1206-1356
Li D, Liu J, Guo B, Liang C, Dang L et al (2016) Osteoclast-derived exosomal MiR-214–3p inhibits osteoblastic bone formation. Nat Commun. https://doi.org/10.1038/ncomms10872
Sun W, Zhao C, Li Y, Wang L, Nie G et al (2016) Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov 2:16015. https://doi.org/10.1038/celldisc.2016.15. https://www.nature.com/celldisc/
Wang X, Guo B, Li Q, Peng J, Yang Z et al (2013) MiR-214 targets ATF4 to inhibit bone formation. Nat Med 19:93–100. https://doi.org/10.1038/nm.3026
Zhao C, Sun W, Zhang P, Ling S, Li Y et al (2015) RNA biology MiR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway MiR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol 12(3):343–353. https://doi.org/10.1080/15476286.2015.1017205
Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y et al (2018) Coupling of bone resorption and formation by RANKL reverse signalling. Nature 561:195–200. https://doi.org/10.1038/s41586-018-0482-7
Ma Q, Liang M, Wu Y, Ding N, Duan L et al (2019) Mature osteoclast-derived apoptotic bodies promote osteogenic differentiation via RANKL-mediated reverse signaling. J Biol Chem 294:11240–11247. https://doi.org/10.1074/jbc.RA119.007625
Liang M, Yin X, Zhang S, Ai H, Luo F et al (2021) Osteoclast-derived small extracellular vesicles induce osteogenic differentiation via inhibiting ARHGAP1. Mol Ther Nucleic Acids 23:1191–1203. https://doi.org/10.1016/j.omtn.2021.01.031
Solberg LB, Stang E, Brorson SH, Andersson G, Reinholt FP (2014) Tartrate-resistant acid phosphatase (TRAP) co-localizes with receptor activator of NF-KB ligand (RANKL) and osteoprotegerin (OPG) in lysosomal-associated membrane protein 1 (LAMP1)-positive vesicles in rat osteoblasts and osteocytes. Histochem Cell Biol 143:195–207. https://doi.org/10.1007/s00418-014-1272-4
Deng L, Wang Y, Peng Y, Wu Y, Ding Y et al (2015) Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone 79:37–42. https://doi.org/10.1016/j.bone.2015.05.022
Chen C, Cheng P, Xie H, De ZH, Wu XP et al (2014) MiR-503 regulates osteoclastogenesis via targeting RANK. J Bone Miner Res 29:338–347. https://doi.org/10.1002/jbmr.2032
Uenaka M, Yamashita E, Kikuta J, Morimoto A, Ao T et al (2022) Osteoblast-derived vesicles induce a switch from bone-formation to bone-resorption in vivo. Nat Commun 13:1–13. https://doi.org/10.1038/s41467-022-28673-2
Ge M, Ke R, Cai T, Yang J, Mu X (2015) Identification and proteomic analysis of osteoblast-derived exosomes. Biochem Biophys Res Commun 467:27–32. https://doi.org/10.1016/j.bbrc.2015.09.135
Zhang P, Mcgrath B, Li S, Frank A, Zambito F et al (2002) The PERK eukaryotic initiation factor 2 kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22:3864–3874. https://doi.org/10.1128/MCB.22.11.3864-3874.2002
Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T et al (2011) Endoplasmic reticulum stress response mediated by the PERK-EIF2α-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem 286:4809–4818. https://doi.org/10.1074/jbc.M110.152900
Cui Y, Luan J, Li H, Zhou X, Han J (2016) Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett 590:185–192. https://doi.org/10.1002/1873-3468.12024
Qin Y, Peng Y, Zhao W, Pan J, Ksiezak-Reding H et al (2017) Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J Biol Chem 292:11021–11033. https://doi.org/10.1074/jbc.M116.770941
Morrell AE, Brown GN, Robinson ST, Sattler RL, Baik AD et al (2018) Mechanically induced Ca2+ oscillations in osteocytes release extracellular vesicles and enhance bone formation. Bone Res 6:1–11. https://doi.org/10.1038/s41413-018-0007-x
Hwang S, Park S-K, Lee HY, Kim SW, Lee JS et al (2014) MiR-140-5p suppresses BMP2-mediated osteogenesis in undifferentiated human mesenchymal stem cells. FEBS Lett 588:2957–2963. https://doi.org/10.1016/j.febslet.2014.05.048
Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW et al (2012) Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res 11:839–849. https://doi.org/10.1021/pr200682z
Ramos TL, Sánchez-Abarca LI, Muntión S, Preciado S, Puig N et al (2016) MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal 14:1–14. https://doi.org/10.1186/s12964-015-0124-8
Narayanan R, Huang C-C, Ravindran S (2016) Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int 2016:3808674. https://doi.org/10.1155/2016/3808674
Zhao L, Jiang S, Hantash BM (2010) Transforming growth factor β1 induces osteogenic differentiation of murine bone marrow stromal cells. Proc Tissue Eng Part A 16:725–733. https://doi.org/10.1089/ten.tea.2009.0495
Luther G, Wagner ER, Zhu G, Kang Q, Luo Q et al (2011) BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Curr Gene Ther 11:229–240. https://doi.org/10.2174/156652311795684777
Qin Y, Wang L, Gao Z, Chen G, Zhang C (2016) Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep 6:1–11. https://doi.org/10.1038/srep21961
Nguyen DX, Bos PD, Massagué J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9:274–284. https://doi.org/10.1038/nrc2622
Urabe F, Patil K, Ramm GA, Ochiya T, Soekmadji C (2021) Extracellular vesicles in the development of organ-specific metastasis. J Extracell Vesicles 10(9):e12125. https://doi.org/10.1002/jev2.12125
Dai J, Escara-Wilke J, Keller JM, Jung Y, Taichman RS et al (2019) Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J Exp Med 216:2883–2899. https://doi.org/10.1084/jem.20190158
Hoshino et al (2015) Tumour exosome integrins determine organotropic metastasis. Nature 176:329–335. https://doi.org/10.1016/j.physbeh.2017.03.040
Gangoda L, Liem M, Ang CS, Keerthikumar S, Adda CG et al (2017) Proteomic profiling of exosomes secreted by breast cancer cells with varying metastatic potential. Proteomics 17:1–5. https://doi.org/10.1002/pmic.201600370
Roodman GD (2009) Mechanisms of bone metastasis. N Engl J Med 350:1655–1664. https://doi.org/10.1056/NEJMRA030831
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593. https://doi.org/10.1038/nrc867
Ye Y, Li SL, Ma YY, Diao YJ, Yang L et al (2017) Exosomal MiR-141-3p regulates osteoblast activity to promote the osteoblastic metastasis of prostate cancer. Oncotarget 8:94834–94849. https://doi.org/10.18632/oncotarget.22014
Hashimoto K, Ochi H, Sunamura S, Kosaka N, Mabuchi Y et al (2018) Cancer-secreted Hsa-MiR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc Natl Acad Sci USA 115:2204–2209. https://doi.org/10.1073/pnas.1717363115
Probert C, Dottorini T, Speakman A, Hunt S, Nafee T et al (2019) Communication of prostate cancer cells with bone cells via extracellular vesicle RNA; a potential mechanism of metastasis. Oncogene 38:1751–1763. https://doi.org/10.1038/s41388-018-0540-5
Yu L, Sui B, Fan W, Lei L, Zhou L et al (2021) Exosomes derived from osteogenic tumor activate osteoclast differentiation and concurrently inhibit osteogenesis by transferring COL1A1-targeting MiRNA-92a-1–5p. J Extracell Vesicles 10(3):e12056. https://doi.org/10.1002/jev2.12056
Yonou H, Ochiai A, Goya M, Kanomata N, Hokama S et al (2004) Intraosseous growth of human prostate cancer in implanted adult human bone: relationship of prostate cancer cell to osteoclasts in osteoblastic metastatic lesions. Prostate 58:406–413. https://doi.org/10.1002/pros.10349
Tiedemann K, Sadvakassova G, Mikolajewicz N, Juhas M, Sabirova Z et al (2019) Exosomal release of L-plastin by breast cancer cells facilitates metastatic bone osteolysis. Transl Oncol 12:462–474. https://doi.org/10.1016/j.tranon.2018.11.014
Loftus A, Cappariello A, George C, Ucci A, Shefferd K et al (2020) Extracellular vesicles from osteotropic breast cancer cells affect bone resident cells. J Bone Miner Res 35:396–412. https://doi.org/10.1002/jbmr.3891
Liu X, Cao M, Palomares M, Wu X, Li A et al (2018) Metastatic breast cancer cells overexpress and secrete MiR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res 20:1–12. https://doi.org/10.1186/s13058-018-1059-y
Taverna S, Pucci M, Giallombardo M, Di Bella MA, Santarpia M et al (2017) Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci Rep 7:1–14. https://doi.org/10.1038/s41598-017-03460-y
Xu Z, Liu X, Wang H, Li J, Dai L et al (2018) Lung adenocarcinoma cell-derived exosomal MiR-21 facilitates osteoclastogenesis. Gene 666:116–122. https://doi.org/10.1016/j.gene.2018.05.008
Sugatani T, Vacher J, Hruska KA (2011) A microRNA expression signature of osteoclastogenesis. Blood 117:3648. https://doi.org/10.1182/BLOOD-2010-10-311415
Valencia K, Luis-Ravelo D, Bovy N, Antón I, Martínez-Canarias S et al (2014) MiRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol 8:689–703. https://doi.org/10.1016/j.molonc.2014.01.012
Li S (2021) The basic characteristics of extracellular vesicles and their potential application in bone sarcomas. J Nanobiotechnol 19:1–12. https://doi.org/10.1186/s12951-021-01028-7
Pan H, Gray R, Braybrooke J, Davies C, Taylor C et al (2017) 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377:1836–1846. https://doi.org/10.1056/nejmoa1701830
Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F et al (2014) Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 7:ra63. https://doi.org/10.1126/scisignal.2005231
Vallabhaneni KC, Penfornis P, Xing F, Hassler Y, Adams KV et al (2017) Stromal cell extracellular vesicular cargo mediated regulation of breast cancer cell metastasis via ubiquitin conjugating enzyme E2 N pathway. Oncotarget 8:109861–109876. https://doi.org/10.18632/oncotarget.22371
Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ et al (2016) Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res 76:5832–5844. https://doi.org/10.1158/0008-5472.CAN-16-1092
Walker ND, Elias M, Guiro K, Bhatia R, Greco SJ et al (2019) Exosomes from differentially activated macrophages influence dormancy or resurgence of breast cancer cells within bone marrow stroma. Cell Death Dis 10(2):59. https://doi.org/10.1038/s41419-019-1304-z
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D et al (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247. https://doi.org/10.1126/science.1153124
Essandoh K, Yang L, Wang X, Huang W, Qin D et al (2015) Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta Mol Basis Dis 1852:2362–2371. https://doi.org/10.1016/j.bbadis.2015.08.010
Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F et al (2013) Neutral sphingomyelinase 2 (NSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem 288:10849–10859. https://doi.org/10.1074/jbc.M112.446831
Yamamoto T, Kosaka N, Ochiya T (2019) Latest advances in extracellular vesicles: from bench to bedside. Sci Technol Adv Mater 20:746–757. https://doi.org/10.1080/14686996.2019.1629835
Mulcahy LA, Pink RC, Carter DRF (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3:24641. https://doi.org/10.3402/jev.v3.24641
Christianson HC, Svensson KJ, Van Kuppevelt TH, Li JP, Belting M (2013) Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci USA 110:17380–17385. https://doi.org/10.1073/pnas.1304266110
Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EA et al (2016) Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J Biol Chem 291:1652–1663. https://doi.org/10.1074/jbc.M115.686295
Marleau AM, Chen CS, Joyce JA, Tullis RH (2012) Exosome removal as a therapeutic adjuvant in cancer. J Transl Med 10:1–12. https://doi.org/10.1186/1479-5876-10-134
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F et al (2012) Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol 227:658–667. https://doi.org/10.1002/jcp.22773
Nishida-Aoki N, Tominaga N, Takeshita F, Sonoda H, Yoshioka Y et al (2017) Disruption of circulating extracellular vesicles as a novel therapeutic strategy against cancer metastasis. Mol Ther 25:181–191. https://doi.org/10.1016/j.ymthe.2016.10.009
Vader P, Mol EA, Pasterkamp G, Schiffelers RM (2016) Extracellular vesicles for drug delivery. Adv Drug Deliv Rev 106:148–156. https://doi.org/10.1016/j.addr.2016.02.006
Ohno SI, Takanashi M, Sudo K, Ueda S, Ishikawa A et al (2013) Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21:185–191. https://doi.org/10.1038/mt.2012.180
Tian Y, Li S, Song J, Ji T, Zhu M et al (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35:2383–2390. https://doi.org/10.1016/j.biomaterials.2013.11.083
Cappariello A, Loftus A, Muraca M, Maurizi A, Rucci N et al (2018) Osteoblast-derived extracellular vesicles are biological tools for the delivery of active molecules to bone. J Bone Miner Res 33:517–533. https://doi.org/10.1002/jbmr.3332
Namee NM, O’Driscoll L (2018) Extracellular vesicles and anti-cancer drug resistance. Biochim Biophys Acta Rev Cancer 1870:123–136. https://doi.org/10.1016/j.bbcan.2018.07.003
Meng-MengXing-Ya LZ, Wei-Xian C, Shan-Liang Z, Hu Q et al (2014) Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumor Biol 35:10773–10779. https://doi.org/10.1007/s13277-014-2377-z
Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M et al (2012) Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS ONE 7(12):e50999. https://doi.org/10.1371/journal.pone.0050999
Maumus M, Rozier P, Boulestreau J, Jorgensen C, Noël D (2020) Mesenchymal stem cell-derived extracellular vesicles: opportunities and challenges for clinical translation. Front Bioeng Biotechnol 8:997. https://doi.org/10.3389/fbioe.2020.00997
Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A et al (2020) Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 29:747–754. https://doi.org/10.1089/scd.2020.0080
Munagala R, Aqil F, Jeyabalan J, Agrawal AK, Mudd AM et al (2017) Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett 393:94–102. https://doi.org/10.1016/J.CANLET.2017.02.004
Somiya M, Yoshioka Y, Ochiya T (2018) Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J Extracell Vesicles 7:1440132. https://doi.org/10.1080/20013078.2018.1440132
Umezu T, Takanashi M, Murakami Y, Ichiro OS, Kanekura K et al (2021) Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol Ther Methods Clin Dev 21:199–208. https://doi.org/10.1016/j.omtm.2021.03.006
Acknowledgements
The present study was supported in part by Project for Cancer Research and Therapeutic Evolution (P-PROMOTE) grant number: 22ama221405h0001 (to Y.Y.) from the Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tamura, T., Yoshioka, Y., Sakamoto, S. et al. Extracellular vesicles in bone homeostasis: key roles of physiological and pathological conditions. J Bone Miner Metab 41, 345–357 (2023). https://doi.org/10.1007/s00774-022-01362-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-022-01362-2