Abstract
Introduction
Bone infections are one of the main reasons for impaired bone regeneration and non-union formation. In previous experimental animal studies we could already demonstrate that bone defects due to prior infections showed a markedly reduced healing capacity, which could effectively be enhanced via application of Wnt3a and Adipose-derived stromal cells (ASCs). For a more in-depth analysis, we investigated proliferation and mineralization of cultured osteoblasts infected with staph aureus and sought to investigate effects of Wnt3a and ASCs on infected osteoblasts.
Materials and methods
Primary murine osteoblasts were isolated from calvariae and infected with staph aureus. Infected osteoblasts received treatment via application of recombinant Wnt3a, ASC conditioned medium and were furthermore cocultured with ASCs. Osteoblasts were evaluated by Alamar blue assay for metabolic activity, TUNEL-assay for apoptosis, ALP and Alizarin Red staining for mineralization. In addition, immunoflourescent staining (IF) and qRT-PCR analyses were performed.
Results
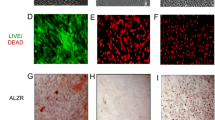
Infected osteoblasts showed a markedly reduced ability for mineralization and increased apoptosis, which could be restored to physiological levels by Wnt3a and ASC treatment. Interestingly, metabolic activity of osteoblasts seemed to be unaffected by staph aureus infection. Additional analyses of Wnt-pathway activity revealed effective enhancement of canonical Wnt-pathway activity in Wnt3a-treated osteoblasts.
Conclusions
In summary, we gained further osteoblast-related insights into pathomechanisms of reduced bone healing capacity upon infections.



Similar content being viewed by others
Abbreviations
- ASC:
-
Adipose-derived stromal cell
- RANKL:
-
Receptor activator nuclear factor-κB ligand
- OPG:
-
Osteoprotegerin
- ALP:
-
Alkaline phospatase
- GSK-3β :
-
Glycogen synthase kinase 3 beta
- sFRP1:
-
Secreted frizzled-related protein 1
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- BMSC:
-
Bone marrow-derived stem cells
- Runx 2:
-
Runt-related transcription factor 2
References
Zimmermann G, Wagner C, Schmeckenbrecher K, Wentzensen A, Moghaddam A (2009) Treatment of tibial shaft non-unions: bone morphogenetic proteins versus autologous bone graft. Injury 40:50–53
Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet 364:369–379. https://doi.org/10.1016/s0140-6736(04)16727-5
Hudson MC, Ramp WK, Nicholson NC, Williams AS, Nousiainen MT (1995) Internalization of Staphylococcus aureus by cultured osteoblasts (in eng). Microb Pathog 19:409–419. https://doi.org/10.1006/mpat.1995.0075
Valour F, Rasigade JP, Trouillet-Assant S, Gagnaire J, Bouaziz A, Karsenty J, Lacour C, Bes M, Lustig S, Bénet T, Chidiac C, Etienne J, Vandenesch F, Ferry T, Laurent F, Lyon BJISG (2015) Delta-toxin production deficiency in Staphylococcus aureus: a diagnostic marker of bone and joint infection chronicity linked with osteoblast invasion and biofilm formation (in eng). Clin Microbiol Infect 21:568.e1. https://doi.org/10.1016/j.cmi.2015.01.026
Bar-Shavit Z (2008) Taking a toll on the bones: regulation of bone metabolism by innate immune regulators (in eng). Autoimmunity 41:195–203. https://doi.org/10.1080/08916930701694469
Claro T, Widaa A, Mc Donell C, Foster TJ, O’brien FJ, Kerrigan SW, (2013) Staphylococcus aureus protein A binding to osteoblast tumour necrosis factor receptor 1 results in activation of nuclear factor kappa B and release of interleukin-6 in bone infection. Microbiology 159:147–154. https://doi.org/10.1099/mic.0.063016-0
Josse J, Guillaume C, Bour C, Lemaire F, Mongaret C, Draux F, Velard F, Gangloff SC (2016) Impact of the maturation of human primary bone-forming cells on their behavior in acute or persistent staphylococcus aureus infection models (in eng). Front Cell Infect Microbiol 6:64–64. https://doi.org/10.3389/fcimb.2016.00064
Bost KL, Ramp WK, Nicholson NC, Bento JL, Marriott I, Hudson MC (1999) Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production (in eng). J Infect Dis 180:1912–1920. https://doi.org/10.1086/315138
Claro T, Widaa A, O’Seaghdha M, Miajlovic H, Foster TJ, O’Brien FJ, Kerrigan SW (2011) Staphylococcus aureus protein A binds to osteoblasts and triggers signals that weaken bone in osteomyelitis. PLoS ONE 6:e18748. https://doi.org/10.1371/journal.pone.0018748
Wagner JM, Zollner H, Wallner C, Ismer B, Schira J, Abraham S, Harati K, Lehnhardt M, Behr B (2016) Surgical debridement is superior to sole antibiotic therapy in a novel murine posttraumatic osteomyelitis model. PLoS ONE 11:e0149389. https://doi.org/10.1371/journal.pone.0149389
Wagner JM, Jaurich H, Wallner C, Abraham S, Becerikli M, Dadras M, Harati K, Duhan V, Khairnar V, Lehnhardt M, Behr B (2017) Diminished bone regeneration after debridement of posttraumatic osteomyelitis is accompanied by altered cytokine levels, elevated B cell activity and increased osteoclast activity (in eng). J Orthop Res. https://doi.org/10.1002/jor.23555
Wagner JM, Reinkemeier F, Wallner C, Dadras M, Huber J, Schmidt SV, Drysch M, Dittfeld S, Jaurich H, Becerikli M, Becker K, Rauch N, Duhan V, Lehnhardt M, Behr B (2019) Adipose derived stromal cells (ASCs) are capable to restore bone regeneration after posttraumatic osteomyelitis and modulate B-cell response. Stem Cells Transl Med doi:DOI. https://doi.org/10.1002/sctm.18-0266
Wagner JM, Reinkemeier F, Dadras M, Wallner C, Huber J, Sogorski A, Sacher M, Schmidt S, Drysch M, Dittfeld S, Becerikli M, Becker K, Rauch N, Lehnhardt M, Behr B (2020) Local Wnt3a treatment restores bone regeneration in large osseous defects after surgical debridement of osteomyelitis (in eng). J Mol Med (Berl). https://doi.org/10.1007/s00109-020-01924-9
Bakker AD, Klein-Nulend J (2012) Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol 816:19–29. https://doi.org/10.1007/978-1-61779-415-5_2
Wallner C, Huber J, Drysch M, Schmidt SV, Wagner JM, Dadras M, Dittfeld S, Becerikli M, Jaurich H, Lehnhardt M, Behr B (2019) Activin receptor 2 antagonization impairs adipogenic and enhances osteogenic differentiation in mouse adipose-derived stem cells and mouse bone marrow-derived stem cells in vitro and in vivo (in eng). Stem Cells Dev 28:384–397. https://doi.org/10.1089/scd.2018.0155
Wallner C, Abraham S, Wagner JM, Harati K, Ismer B, Kessler L, Zollner H, Lehnhardt M, Behr B (2016) Local application of isogenic adipose-derived stem cells restores bone healing capacity in a type 2 diabetes model (in eng). Stem Cells Transl Med 5:836–844. https://doi.org/10.5966/sctm.2015-0158
J E, S R, W R, (1999) Mechanisms of S aureus Invasion of cultured osteoblasts. Microb Pathog 26:317–323
Behr B, Ko SH, Wong VW, Gurtner GC, Longaker MT (2010) Stem cells. (in eng). Plast Reconstr Surg 126:1163–1171. https://doi.org/10.1097/PRS.0b013e3181ea42bb
Chen Q, Hou T, Luo F, Wu X, Xie Z, Xu J (2014) Involvement of toll-like receptor 2 and pro-apoptotic signaling pathways in bone remodeling in osteomyelitis (in eng). Cell Physiol Biochem 34:1890–1900. https://doi.org/10.1159/000366387
Sanchez CJ Jr, Ward CL, Romano DR, Hurtgen BJ, Hardy SK, Woodbury RL, Trevino AV, Rathbone CR, Wenke JC (2013) Staphylococcus aureus biofilms decrease osteoblast viability, inhibits osteogenic differentiation, and increases bone resorption in vitro (in eng). BMC Musculoskelet Disord 14:187. https://doi.org/10.1186/1471-2474-14-187
Tang C-H, Kassem A, Lindholm C, Lerner UH (2016) Toll-Like receptor 2 stimulation of osteoblasts mediates S. Aureus induced bone resorption and osteoclastogenesis through enhanced rankl. PLoS ONE 11:e0156708. https://doi.org/10.1371/journal.pone.0156708
Somayaji SN, Huet YM, Gruber HE, Hudson MC (2010) UV-killed S. Aureus enhances adhesion and differentiation of osteoblasts on bone-associated biomaterials (in eng). J Biomed Mater Res A 95:574–579. https://doi.org/10.1002/jbm.a.32890
Jin T, Lu Y, He QX, Wang H, Li BF, Zhu LY, Xu QY (2015) The role of microrNA, miR-24, and its target CHI3L1 in osteomyelitis caused by S. Aureus (in eng). J Cell Biochem 116:2804–2813. https://doi.org/10.1002/jcb.25225
Tucker KA, Reilly SS, Leslie CS, Hudson MC (2000) Intracellular S. Aureus induces apoptosis in mouse osteoblasts (in eng). FEMS Microbiol Lett 186:151–156. https://doi.org/10.1111/j.1574-6968.2000.tb09096.x
Young AB, Cooley ID, Chauhan VS, Marriott I (2011) Causative agents of osteomyelitis induce death domain-containing TNF-related apoptosis-inducing ligand receptor expression on osteoblasts (in eng). Bone 48:857–863. https://doi.org/10.1016/j.bone.2010.11.015
Neumann J, Endermann T, Ehlers S, Reiling N (2009) Inverse relationship of TLR/NF-κB signalling and the Wnt/β-catenin pathway during inflammation: deciphering the role of Frizzled1 in M. tuberculosis infection. Cell Commun Signal 7:A52. https://doi.org/10.1186/1478-811X-7-S1-A52
Neumann J, Schaale K, Farhat K, Endermann T, Ulmer AJ, Ehlers S, Reiling N (2010) Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. (in eng). FASEB J 24:4599–4612. https://doi.org/10.1096/fj.10-160994
Widaa A, Claro T, Foster TJ, O’Brien FJ, Kerrigan SW (2012) Staphylococcus aureus protein A plays a critical role in mediating bone destruction and bone loss in osteomyelitis. PLoS ONE 7:e40586. https://doi.org/10.1371/journal.pone.0040586
Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation (in eng). Dev Cell 8:751–764. https://doi.org/10.1016/j.devcel.2005.02.017
Friedman MS, Oyserman SM, Hankenson KD (2009) Wnt11 promotes osteoblast maturation and mineralization through R-spondin 2 (in eng). J Biol Chem 284:14117–14125. https://doi.org/10.1074/jbc.M808337200
Tresguerres FGF, Torres J, López-Quiles J, Hernández G, Vega JA, Tresguerres IF (2020) The osteocyte: a multifunctional cell within the bone (in eng). Ann Anat 227:151422. https://doi.org/10.1016/j.aanat.2019.151422
Franz-Odendaal TA, Hall BK, Witten PE (2006) Buried alive: how osteoblasts become osteocytes (in eng). Dev Dyn 235:176–190. https://doi.org/10.1002/dvdy.20603
Hanada R, Hanada T, Sigl V, Schramek D, Penninger JM (2011) RANKL/RANK-beyond bones (in eng). J Mol Med (Berl) 89:647–656. https://doi.org/10.1007/s00109-011-0749-z
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression (in eng). Nat Med 17:1231–1234. https://doi.org/10.1038/nm.2452
Bodine PV, Billiard J, Moran RA, Ponce-de-Leon H, McLarney S, Mangine A, Scrimo MJ, Bhat RA, Stauffer B, Green J, Stein GS, Lian JB, Komm BS (2005) The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis (in eng). J Cell Biochem 96:1212–1230. https://doi.org/10.1002/jcb.20599
Yao W, Cheng Z, Shahnazari M, Dai W, Johnson ML, Lane NE (2010) Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects (in eng). J Bone Miner Res 25:190–199. https://doi.org/10.1359/jbmr.090719
Häusler KD, Horwood NJ, Chuman Y, Fisher JL, Ellis J, Martin TJ, Rubin JS, Gillespie MT (2004) Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation (in eng). J Bone Miner Res 19:1873–1881. https://doi.org/10.1359/jbmr.040807
Wang Y, Volloch V, Pindrus MA, Blasioli DJ, Chen J, Kaplan DL (2007) Murine osteoblasts regulate mesenchymal stem cells via WNT and cadherin pathways: mechanism depends on cell-cell contact mode (in eng). J Tissue Eng Regen Med 1:39–50. https://doi.org/10.1002/term.6
Rinker TE, Hammoudi TM, Kemp ML, Lu H, Temenoff JS (2014) Interactions between mesenchymal stem cells, adipocytes, and osteoblasts in a 3D tri-culture model of hyperglycemic conditions in the bone marrow microenvironment (in eng). Integr Biol 6:324–337. https://doi.org/10.1039/c3ib40194d
Chung YS, Baylink DJ, Srivastava AK, Amaar Y, Tapia B, Kasukawa Y, Mohan S (2004) Effects of secreted frizzled-related protein 3 on osteoblasts in vitro (in eng). J Bone Miner Res 19:1395–1402. https://doi.org/10.1359/jbmr.040412
Khamees N, Hill DJ, Kafienah W (2020) Mechanisms of interaction of S. Aureus with human mesenchymal stem cells and their differentiated phenotypes. bioRxiv. 14:184. https://doi.org/10.1101/2020.01.09.900373
Acknowledgements
This work was supported by a grant of the DFG (Deutsche Forschungsgemeinschaft) (BE 4169/8-1).
Author information
Authors and Affiliations
Contributions
JW, YS, SD, and MB did all the experiments and BB, JW, YS, FR, MD, CW, JH, AS, MS, SD, MB, ML interpreted data and contributed to research design. All authors have read and accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
Isolation of murine osteoblasts was performed according to the guidelines of the National Institute of Health for the use of experimental animals and after approval by the German legislation. The protocol was approved by the LANUV (NRW, Germany; Permit-Number: 84–02.04.2014.A044).
Informed consent
Informed consent is not applicable for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Wagner, J.M., Steubing, Y., Dadras, M. et al. Wnt3a and ASCs are capable of restoring mineralization in staph aureus-infected primary murine osteoblasts. J Bone Miner Metab 40, 20–28 (2022). https://doi.org/10.1007/s00774-021-01269-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-021-01269-4