Abstract
Introduction
Collagen from marine esponges has been used as a promising material for tissue engineering proposals. Similarly, photobiomodulation (PBM) is able of modulating inflammatory processes after an injury, accelerating soft and hard tissue healing and stimulating neoangiogenesis. However, the effects of the associated treatments on bone tissue healing have not been studied yet. In this context, the present study aimed to evaluate the biological temporal modifications (using two experimental periods) of marine sponge collagen or sponging (SPG) based scaffold and PBM on newly formed bone using a calvaria bone defect model.
Material and Methods
Wistar rats were distributed into two groups: SPG or SPG/PBM and euthanized into two different experimental periods (15 and 45 days post-surgery). A cranial critical bone defect was used to evaluate the effects of the treatments. Histology, histomorfometry and immunohistological analysis were performed.
Results
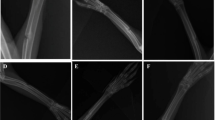
Histological findings demonstrated that SPG/PBM-treated animals, 45 days post-surgery, demonstrated a higher amount of connective and newly formed bone tissue at the region of the defect compared to CG. Notwithstanding, no difference among groups were observed in the histomorphometry. Interestingly, for both anti-transforming growth factor-beta (TGF-β) and anti-vascular endothelial growth factor (VEGF) immunostaining, higher values for SPG/PBM, at 45 days post-surgery could be observed.
Conclusion
It can be concluded that the associated treatment can be considered as a promising therapeutical intervention.






Similar content being viewed by others
References
Blais M, Parenteau-Bareil R, Cadau S, Berthod F (2013) Concise review: tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl Med 2:545–551
Pozzolini M, Scarfì S, Gallus L, Castellano M, Vicini S, Cortese K et al (1847) Production, characterization and biocompatibility evaluation of collagen membranes derived from marine sponge chondrosia reniformis Nardo. Mar Drugs 2018:111–131
Silva TH, Moreira-Silva J, Marques ALP, Domingues A, Bayon Y, Reis RL (2014) Marine origin collagens and its potential applications. Marine Drugs 16:5881–5901
Lin Z, Solomon KL, Zhang X, Pavlos NJ, Abel T, Willers C et al (2011) In vitro evaluation of natural marine sponge collagen as a scaffold for bone tissue engineering. Int J Biol Sci 7:968–977
Zdarta J, Norman M, Smułek W, Moszyński D, Kaczorek E, Stelling AL et al (2017) Spongin-based scaffolds from Hippospongia communis demosponge as an effective support for lipase immobilization. Catalysts 7:147–167
Widdowson JP, Picton AJ, Vince V, Wright CJ, Mearns-Spragg A (2018) In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. J Biomed Mater Res Part B Appl Biomater 106:1524–1533
Parisi JR, Avanzi IR, Santana AF, Renno ACM, Andrade AL, Fortulan CA et al (2018) Incorporation of collagen from marine sponges (spongin) into hydroxyapatite samples: characterization and in vitro biological evaluation. Mar Biotechnol 21:30–37
Exposito JY, Cluzel C, Garrone R, Lethias C (2002) Evolution of collagens. Anat Rec 268:302–316
Green D, Howard D, Yang X, Kelly M, Oreffo ROCOC (2003) Natural marine sponge fiber skeleton: a biomimetic scaffold. Tissue Eng 9:1156–1166
Iwatsubo T, Kishi R, Miura T, Ohzono T, Yamaguchi T (2015) Formation of hydroxyapatite skeletal materials from hydrogel matrices via artificial biomineralization. J Phys Chem B 119:8793–8799
Tim CR, Pinto KNZ, Rossi BRO, Fernandes K, Matsumoto MA, Parizotto NA et al (2014) Low-level laser therapy enhances the expression of osteogenic factors during bone repair in rats. Lasers Med Sci 29:147–156
Magri AMP, Fernandes KR, Assis L, Mendes NA, da Silva Santos ALY, de Oliveira DE et al (2015) Photobiomodulation and bone healing in diabetic rats: evaluation of bone response using a tibial defect experimental model. Lasers Med Sci 30:1949–1957
Hamblin MR (2018) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 94:199–212
Diniz IMA, Carreira ACO, Sipert CR, Uehara CM, Moreira MSN, Freire L et al (2018) Photobiomodulation of mesenchymal stem cells encapsulated in an injectable rhBMP4-loaded hydrogel directs hard tissue bioengineering. J Cell Physiol 233:4907–4918
Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R et al (2014) Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury [Internet] 45:S3–7. https://doi.org/10.1016/j.injury.2014.04.002
Ruh AC, Frigo L, Cavalcanti MFXB, Svidnicki P, Vicari VN, Lopes-Martins RAB et al (2018) Laser photobiomodulation in pressure ulcer healing of human diabetic patients: gene expression analysis of inflammatory biochemical markers. Lasers Med Sci. 33:165–171
Karu T (2013) Is it time to consider photobiomodulation as a drug equivalent? Photomed Laser Surg 31:189–191
Huang YY, Chen ACH, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level lightherapy. Dose Response 7:358–383
Hamblin MR, Huang YY, Sharma SK, Carroll J (2011) Biphasic dose response in low level light therapy—an update. Dose Response 9:602–618
Naeser MA, Hamblin MR (2011) Potential for transcranial laser or LED therapy to treat stroke, traumatic brain injury, and neurodegenerative disease. Photomed Laser Surg 29:443–446
de Almeida TG, Alves MBR, Batissaco L, Torres MA, de Andrade AFC, Mingoti RD et al (2019) Does low-level laser therapy on degenerated ovine testes improve post-thawed sperm characteristics? Lasers Med Sci 34:1001–1009
Ravera S, Ferrando S, Agas D, De Angelis N, Raffetto M, Sabbieti MG et al (2019) 1064 nm Nd:YAG laser light affects transmembrane mitochondria respiratory chain complexes. J Biophotonics 12:1–7
Bossini PS, Muniz Renno AC, Ribeiro DA, Fangel R, Peitl O, Zanotto ED et al (2011) Biosilicate® and low-level laser therapy improve bone repair in osteoporotic rats. J Tissue Eng Regen Med 5:229–237
de Andrade ALM, Parisi JR, Parizotto NA, Luna GF, de Oliveira Leal ÂM, Brassolatti P et al (2018) Photobiomodulation effect on the proliferation of adipose tissue mesenchymal stem cells. Lasers Med Sci 34:677–683
Fávaro-Pípi E, Ribeiro DA, Ribeiro JU, Bossini P, Oliveira P, Parizotto NA et al (2011) Low-level laser therapy induces differential expression of osteogenic genes during bone repair in rats. Photomed Laser Surg 29:311–317
Tim CR, Bossini PS, Kido HW, Malavazi I, von Zeska Kress MR, Carazzolle MF et al (2016) Low-level laser therapy induces an upregulation of collagen gene expression during the initial process of bone healing: a microarray analysis. J Biomed Opt 21:088001–88009
Swatschek D, Schatton W, Kellermann J, Müller WEG, Kreuter J (2002) Marine sponge collagen: isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur J Pharm Biopharm 53:107–113
Özel T, Bártolo PJ, Ceretti E, De Ciurana Gay JD, Rodríguez CA, Da Silva JVL (2016) Biomedical devices: design, prototyping, and manufacturing. Wiley, Hoboken, pp 1–190
Lopez-Heredia MA, Sa Y, Salmon P, De Wijn JR, Wolke JGC, Jansen JA (2012) Bulk properties and bioactivity assessment of porous polymethylmethacrylate cement loaded with calcium phosphates under simulated physiological conditions. Acta Biomater 8:3120–3127
Wang L, Yoon DM, Spicer PP, Henslee AM, Scott DW, Wong ME et al (2013) Characterization of porous polymethylmethacrylate space maintainers for craniofacial reconstruction. J Biomed Mater Res Part B Appl Biomater 101:813–825
Magri AMP, Fernandes KR, Kido HW, Fernandes GS, de Fermino S, Gabbai-Armelin PR, et al (2019) Bioglass/PLGA associated to photobiomodulation: effects on the healing process in an experimental model of calvarial bone defect. J Mater Sci Mater Med [Internet] 30:105–114. https://doi.org/10.1007/s10856-019-6307-x
Tim CR, Bossini PS, Kido HW, Malavazi I, von Zeska Kress MR, Carazzolle MF et al (2015) Effects of low-level laser therapy on the expression of osteogenic genes during the initial stages of bone healing in rats: a microarray analysis. Lasers Med Sci 30:2325–2333
Sarvestani FK, Dehno NS, Nazhvani SD, Bagheri MH, Abbasi S, Khademolhosseini Y et al (2017) Effect of low-level laser therapy on fracture healing in rabbits effect of low-level laser therapy on fracture healing in rabbits. Laser Ther 26:189–193
de Freitas NR, Guerrini LB, Esper LA, Sbrana MC, da Dalben G, Soares S et al (2018) Evaluation of photobiomodulation therapy associated with guided bone regeneration in critical size defects. In vivo study. J Appl Oral Sci 26:0244–255
de Oliveira LSS, de Araújo AA, de Araújo Júnior RF, Barboza CAG, Borges BCD, da Silva JSP (2017) Low-level laser therapy (780 nm) combined with collagen sponge scaffold promotes repair of rat cranial critical-size defects and increases TGF-β, FGF-2, OPG/RANK and osteocalcin expression. Int J Exp Pathol 98:75–85
Majidinia M, Sadeghpour A, Yousefi B (2018) The roles of signaling pathways in bone repair and regeneration. J Cell Physiol 233:2937–2948
Yang YQ, Tan YY, Wong R, Wenden A, Zhang LK, Rabie ABM (2012) The role of vascular endothelial growth factor in ossification. Int J Oral Sci 4:64–68
Zhou Y, Huang R, Fan W, Prasadam I, Crawford R, Xiao Y (2018) Mesenchymal stromal cells regulate the cell mobility and the immune response during osteogenesis through secretion of vascular endothelial growth factor A. J Tissue Eng Regen Med 12:e566–e578
Hosseinpour S, Fekrazad R, Arany PR, Ye Q (2019) Molecular impacts of photobiomodulation on bone regeneration: a systematic review. Prog Biophys Mol Biol 4:005–18
Acknowledgements
Authors would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the scholarship (grant no. 2016/13636-9). This research was supported by fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Animal Care Committee guidelines of the Federal University of São Paulo (CEUA no. 4331220318) approved the present study and national guidelines for the care and use of laboratory animals were observed.
Informed consent
No informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Cruz, M.A., Fernandes, K.R., Parisi, J.R. et al. Marine collagen scaffolds and photobiomodulation on bone healing process in a model of calvaria defects. J Bone Miner Metab 38, 639–647 (2020). https://doi.org/10.1007/s00774-020-01102-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01102-4