Abstract
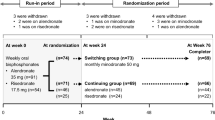
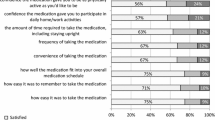
Minodronate is a potent nitrogen-containing bisphosphonate that can be administered according to a monthly (every 4 weeks) dosing regimen. A 6-month, cluster-randomized, open-label, multicenter, crossover trial was conducted to test the preference of Japanese patients with osteoporosis for monthly bisphosphonate versus weekly bisphosphonate. One hundred and forty-seven patients (postmenopausal women and men) with primary osteoporosis were recruited at eight outpatient clinics. The clinics were randomized into two groups according to the dosing protocol—monthly minodronate followed by weekly alendronate or risedronate for a total of 24 weeks, or weekly alendronate or risedronate followed by monthly minodronate for 24 weeks. Patient preference for either the monthly or weekly bisphosphonate regimen was evaluated using a preference questionnaire. One hundred and fifteen patients (78.2 %) who completed the trial were processed for the analyses. Significantly more patients preferred the monthly bisphosphonate regimen (65.2 %) than the weekly bisphosphonate regimen (15.7 %) (P = 0.007). ‘Dosing schedule fits lifestyle better’ was the most common reason given for the patient preference for both the monthly (32.0 %) and weekly bisphosphonate (33.3 %) regimens. Significantly more patients found the monthly bisphosphonate regimen to be more convenient (73.0 %) than the weekly bisphosphonate regimen (13.9 %) (P < 0.0001). The safety profiles of the two regimens were similar. The present trial demonstrated a strong patient preference for and the convenience of the monthly bisphosphonate regimen over the weekly bisphosphonate regimen in Japanese patients with osteoporosis.







Similar content being viewed by others
References
Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD (2012) Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 166:735–741
Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Morii H, Ohashi Y, Nakamura T (2009) Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int 20:1429–1437
Okazaki R, Hagino H, Ito M, Sone T, Nakamura T, Mizunuma H, Fukunaga M, Shiraki M, Nishizawa Y, Ohashi Y, Matsumoto T (2012) Efficacy and safety of monthly oral minodronate in patients with involutional osteoporosis. Osteoporos Int 23:1737–1745
Emkey R, Koltun W, Beusterien K, Seidman L, Kivitz A, Devas V, Masanauskaite D (2005) Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO). Curr Med Res Opin 21:1895–1903
Hadji P, Minne H, Pfeifer M, Bourgeois P, Fardellone P, Licata A, Devas V, Masanauskaite D, Barrett-Connor E (2008) Treatment preference for monthly oral ibandronate and weekly oral alendronate in women with postmenopausal osteoporosis: a randomized, crossover study (BALTO II). Joint Bone Spine 75:303–310
Payer J, Killinger Z, Sulková I, Celec P (2008) Preferences of patients receiving bisphosphonates—how to influence the therapeutic adherence. Biomed Pharmacother 62:122–124
Payer J, Cierny D, Killinger Z, Sulková I, Behuliak M, Celec P (2009) Preferences of patients with post-menopausal osteoporosis treated with bisphosphonates—the VIVA II study. J Int Med Res 37:1225–1259
Kastelan D, Lozo P, Stamenkovic D, Miskic B, Vlak T, Kolak Z, Milas Ahic J, Altabas V, Crncevic Orlic Z, Korsic M (2009) Preference for weekly and monthly bisphosphonates among patients with postmenopausal osteoporosis: results from the Croatian PROMO Study. Clin Rheumatol 28:321–326
Makita K, Okano H, Furuya T, Urano T, Hirabayashi H, Kumakubo T, Iwamoto J (2014) Survey of the utility of once-monthly bisphosphonate treatment for improvement of medication adherence in osteoporosis patients in Japan. J Bone Miner Metab 33:55–60
Orimo H, Sugioka Y, Fukunaga M, Muto Y, Hotokebuchi T, Gorai I, Nakamura T, Kushida K, Tanaka H, Ikai T, Ohhashi Y (1998) Diagnostic criteria of primary osteoporosis. J Bone Miner Metab 16:139–150
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H, Osteoporosis Diagnostic Criteria Review Committee: Japanese Society for Bone and Mineral Research (2001) Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 19:331–337
Fujiwara S, Sone T, Yamazaki K, Yoshimura N, Nakatsuka K, Masunari N, Fujita S, Kushida K, Fukunaga M (2005) Heel bone ultrasound predicts non-spine fracture in Japanese men and women. Osteoporos Int 16:2107–2112
Wüster C, Heilmann P, Pereira-Lima J, Schlegel J, Anstätt K, Soballa T (1998) Quantitative ultrasonometry (QUS) for the evaluation of osteoporosis risk: reference data for various measurement sites, limitations and application possibilities. Exp Clin Endocrinol Diabetes 106:277–288
Uchida S, Taniguchi T, Shimizu T, Kakikawa T, Okuyama K, Okaniwa M, Arizono H, Nagata K, Santora AC, Shiraki M, Fukunaga M, Tomomitsu T, Ohashi Y, Nakamura T (2005) Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab 23:382–388
Kishimoto H, Fukunaga M, Kushida K, Shiraki M, Itabashi A, Nawata H, Nakamura T, Ohta H, Takaoka K, Ohashi Y, Risedronate Phase III Research Group (2006) Efficacy and tolerability of once-weekly administration of 17.5 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with 2.5 mg once-daily dosage regimen. J Bone Miner Metab 24:405–413
Sampalis JS, Adachi JD, Rampakakis E, Vaillancourt J, Karellis A, Kindundu C (2012) Long-term impact of adherence to oral bisphosphonates on osteoporotic fracture incidence. J Bone Miner Res 27:202–210
Cotté FE, Fardellone P, Mercier F, Gaudin AF, Roux C (2010) Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int 21:145–155
Rizzoli R, Reginster JY, Boonen S, Bréart G, Diez-Perez A, Felsenberg D, Kaufman JM, Kanis JA, Cooper C (2011) Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int 89:91–104
Iwamoto J (2014) Monthly risedronate for the treatment of postmenopausal osteoporosis. Pharm Anal Acta 5:285
Acknowledgments
This study was supported by The Waksman Foundation of Japan INC.
Conflict of interest
We have no conflict of interest and disclosure.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Iwamoto, J., Okano, H., Furuya, T. et al. Patient preference for monthly bisphosphonate versus weekly bisphosphonate in a cluster-randomized, open-label, crossover trial: Minodroate Alendronate/Risedronate Trial in Osteoporosis (MARTO). J Bone Miner Metab 34, 201–208 (2016). https://doi.org/10.1007/s00774-015-0653-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0653-7