Abstract
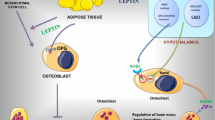
Adipose tissue has been more accepted as an active contributor to whole body homeostasis, rather than just a fat depot, since leptin, a 16 kDa protein, was discovered as the product of the obese gene in 1994. With more and more studies conducted on this hormone, it has been shown that there is a close relationship between adipose tissue and bone, which have important effects on each other. Bone is the source of many hormones, such as osteocalcin, that can affect energy metabolism and then the anabolism or catabolism of fat tissue. In contrast, the adipose tissue synthesizes and releases a series of adipokines, which are involved in bone metabolism through direct or indirect effects on bone formation and resorption. Interestingly, leptin, one of the most important cytokines derived from fat tissue, seems to account for the largest part of effects on bone, through direct or indirect involvement in bone remodeling and by playing a significant role in many bone diseases, such as osteoporosis, osteoarthritis, rheumatic arthritis, bone tumors and even fractures. In this review, we will discuss the progress in leptin research, particularly focusing on the roles of leptin in bone diseases.

Similar content being viewed by others
References
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP (2007) Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides 28:1012–1019
Hamrick MW, Pennington C, Newton D, Xie D, Isales C (2004) Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34:376–383
Parm AL, Jurimae J, Saar M, Parna K, Tillmann V, Maasalu K, Neissaar I, Jurimae T (2011) Plasma adipocytokine and ghrelin levels in relation to bone mineral density in prepubertal rhythmic gymnasts. J Bone Miner Metab 29:717–724
Hamrick MW, Ferrari SL (2008) Leptin and the sympathetic connection of fat to bone. Osteoporos Int 19:905–912
Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83:1263–1271
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G (2005) Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520
Karsenty G (2006) Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 4:341–348
Schwetz V, Pieber T, Obermayer-Pietsch B (2012) The endocrine role of the skeleton: background and clinical evidence. Eur J Endocrinol 166:959–967
Benovic JL, Bouvier M, Caron MG, Lefkowitz RJ (1988) Regulation of adenylyl cyclase-coupled beta-adrenergic receptors. Annu Rev Cell Biol 4:405–428
Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G (2005) The molecular clock mediates leptin-regulated bone formation. Cell 122:803–815
Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G (2004) ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin–Lowry syndrome. Cell 117:387–398
Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649
Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S (1998) Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393:72–76
Karsenty G, Oury F (2010) The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab 95:4795–4801
Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G (2009) A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138:976–989
Hanada R, Teranishi H, Pearson JT, Kurokawa M, Hosoda H, Fukushima N, Fukue Y, Serino R, Fujihara H, Ueta Y, Ikawa M, Okabe M, Murakami N, Shirai M, Yoshimatsu H, Kangawa K, Kojima M (2004) Neuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nat Med 10:1067–1073
Baldock PA, Allison S, McDonald MM, Sainsbury A, Enriquez RF, Little DG, Eisman JA, Gardiner EM, Herzog H (2006) Hypothalamic regulation of cortical bone mass: opposing activity of Y2 receptor and leptin pathways. J Bone Miner Res 21:1600–1607
Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H (2002) Hypothalamic Y2 receptors regulate bone formation. J Clin Investig 109:915–921
Brighton PJ, Szekeres PG, Willars GB (2004) Neuromedin U and its receptors: structure, function, and physiological roles. Pharmacol Rev 56:231–248
Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, Iwasaki M, Inose H, Ida T, Mieda M, Takeuchi Y, Fukumoto S, Fujita T, Kato S, Kangawa K, Kojima M, Shinomiya K, Takeda S (2007) Central control of bone remodeling by neuromedin U. Nat Med 13:1234–1240
Howard AD, Wang R, Pong SS, Mellin TN, Strack A et al (2000) Identification of receptors for neuromedin U and its role in feeding. Nature 406:70–74
Wren AM, Small CJ, Abbott CR, Jethwa PH, Kennedy AR, Murphy KG, Stanley SA, Zollner AN, Ghatei MA, Bloom SR (2002) Hypothalamic actions of neuromedin U. Endocrinology 143:4227–4234
Cirmanova V, Bayer M, Starka L, Zajickova K (2008) The effect of leptin on bone: an evolving concept of action. Physiol Res 57 Suppl 1:S143–S151
Lin S, Boey D, Herzog H (2004) NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides 38:189–200
Baldock PA, Lee NJ, Driessler F, Lin S, Allison S et al (2009) Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS ONE 4:e8415
Lee NJ, Herzog H (2009) NPY regulation of bone remodelling. Neuropeptides 43:457–463
Teixeira L, Sousa DM, Nunes AF, Sousa MM, Herzog H, Lamghari M (2009) NPY revealed as a critical modulator of osteoblast function in vitro: new insights into the role of Y1 and Y2 receptors. J Cell Biochem 107:908–916
Shi YC, Baldock PA (2012) Central and peripheral mechanisms of the NPY system in the regulation of bone and adipose tissue. Bone 50:430–436
Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A et al (1995) The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377:530–532
Allison SJ, Baldock PA, Herzog H (2007) The control of bone remodeling by neuropeptide Y receptors. Peptides 28:320–325
Baldock PA, Sainsbury A, Allison S, Lin EJ, Couzens M, Boey D, Enriquez R, During M, Herzog H, Gardiner EM (2005) Hypothalamic control of bone formation: distinct actions of leptin and y2 receptor pathways. J Bone Miner Res 20:1851–1857
Flier JS (1997) Leptin expression and action: new experimental paradigms. Proc Natl Acad Sci USA 94:4242–4245
Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP, Koniaris A (2011) Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab 301:E567–E584
Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL (1999) Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140:1630–1638
Hess R, Pino AM, Rios S, Fernandez M, Rodriguez JP (2005) High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. J Cell Biochem 94:50–57
Reseland JE, Syversen U, Bakke I, Qvigstad G, Eide LG, Hjertner O, Gordeladze JO, Drevon CA (2001) Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res 16:1426–1433
Astudillo P, Rios S, Pastenes L, Pino AM, Rodriguez JP (2008) Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J Cell Biochem 103:1054–1065
Gordeladze JO, Drevon CA, Syversen U, Reseland JE (2002) Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem 85:825–836
Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC (2002) Leptin inhibits osteoclast generation. J Bone Miner Res 17:200–209
Burguera B, Hofbauer LC, Thomas T, Gori F, Evans GL, Khosla S, Riggs BL, Turner RT (2001) Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology 142:3546–3553
Martin A, de Vittoris R, David V, Moraes R, Begeot M, Lafage-Proust MH, Alexandre C, Vico L, Thomas T (2005) Leptin modulates both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats. Endocrinology 146:3652–3659
Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA (2005) Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res 20:994–1001
Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, Iwaniec UT (2013) Peripheral leptin regulates bone formation. J Bone Miner Res 28:22–34
Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG Jr, Karsenty G (2008) Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci USA 105:20529–20533
Figenschau Y, Knutsen G, Shahazeydi S, Johansen O, Sveinbjornsson B (2001) Human articular chondrocytes express functional leptin receptors. Biochem Biophys Res Commun 287:190–197
Kishida Y, Hirao M, Tamai N, Nampei A, Fujimoto T, Nakase T, Shimizu N, Yoshikawa H, Myoui A (2005) Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification. Bone 37:607–621
Maor G, Rochwerger M, Segev Y, Phillip M (2002) Leptin acts as a growth factor on the chondrocytes of skeletal growth centers. J Bone Miner Res 17:1034–1043
Hebebrand J, Muller TD, Holtkamp K, Herpertz-Dahlmann B (2007) The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry 12:23–35
Katsiki N, Mikhailidis DP, Gotzamani-Psarrakou A, Yovos JG, Karamitsos D (2011) Effect of various treatments on leptin, adiponectin, ghrelin and neuropeptide Y in patients with type 2 diabetes mellitus. Expert Opin Ther Targets 15:401–420
Hou N, Luo JD (2011) Leptin and cardiovascular diseases. Clin Exp Pharmacol Physiol 38:905–913
Zhou R, Deng J, Zhang M, Zhou HD, Wang YJ (2011) Association between bone mineral density and the risk of Alzheimer’s disease. J Alzheimer’s Dis 24:101–108
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Investig 115:3318–3325
Thomas T, Burguera B, Melton LJ 3rd, Atkinson EJ, O’Fallon WM, Riggs BL, Khosla S (2001) Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone 29:114–120
Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K (2001) Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol 55:341–347
Grigorie D, Neacsu E, Marinescu M, Popa O (2003) Circulating osteoprotegerin and leptin levels in postmenopausal women with and without osteoporosis. Roman J Int Med 41:409–415
Roux C, Arabi A, Porcher R, Garnero P (2003) Serum leptin as a determinant of bone resorption in healthy postmenopausal women. Bone 33:847–852
Sebastian-Ochoa A, Fernandez-Garcia D, Reyes-Garcia R, Mezquita-Raya P, Rozas-Moreno P, Alonso-Garcia G, Munoz-Torres M (2012) Adiponectin and leptin serum levels in osteoporotic postmenopausal women treated with raloxifene or alendronate. Menopause (New York, NY) 19:172–177
Hipmair G, Bohler N, Maschek W, Soriguer F, Rojo-Martinez G, Schimetta W, Pichler R (2010) Serum leptin is correlated to high turnover in osteoporosis. Neuro Endocrinol Lett 31:155–160
Di Carlo C, Tommaselli GA, Gargano V, Sammartino A, Bifulco G, Tauchmanova L, Colao A, Nappi C (2007) Effects of estrogen-progestin therapy on serum levels of RANKL, osteoprotegerin, osteocalcin, leptin, and ghrelin in postmenopausal women. Menopause (New York, NY) 14:38–44
Sato M, Takeda N, Sarui H, Takami R, Takami K, Hayashi M, Sasaki A, Kawachi S, Yoshino K, Yasuda K (2001) Association between serum leptin concentrations and bone mineral density, and biochemical markers of bone turnover in adult men. J Clin Endocrinol Metab 86:5273–5276
Ormarsdottir S, Ljunggren O, Mallmin H, Olofsson H, Blum WF, Loof L (2001) Inverse relationship between circulating levels of leptin and bone mineral density in chronic liver disease. J Gastroenterol Hepatol 16:1409–1414
Fontana L, Shew JL, Holloszy JO, Villareal DT (2005) Low bone mass in subjects on a long-term raw vegetarian diet. Arch Intern Med 165:684–689
Pobeha P, Ukropec J, Skyba P, Ukropcova B, Joppa P, Kurdiova T, Javorsky M, Klimes I, Tkac I, Gasperikova D, Tkacova R (2011) Relationship between osteoporosis and adipose tissue leptin and osteoprotegerin in patients with chronic obstructive pulmonary disease. Bone 48:1008–1014
Odabasi E, Ozata M, Turan M, Bingol N, Yonem A, Cakir B, Kutlu M, Ozdemir IC (2000) Plasma leptin concentrations in postmenopausal women with osteoporosis. Eur J Endocrinol 142:170–173
Ruhl CE, Everhart JE (2002) Relationship of serum leptin concentration with bone mineral density in the United States population. J Bone Miner Res 17:1896–1903
Hadji P, Bock K, Gotschalk M, Hars O, Backhus J, Emons G, Schulz KD (2003) The influence of serum leptin concentration on bone mass assessed by quantitative ultrasonometry in pre and postmenopausal women. Maturitas 44:141–148
Sahin G, Polat G, Baethis S, Milcan A, Baethdatoethlu O, Erdoethan C, Camdeviren H (2003) Body composition, bone mineral density, and circulating leptin levels in postmenopausal Turkish women. Rheumatol Int 3:87–91
Wu N, Wang QP, Li H, Wu XP, Sun ZQ, Luo XH (2010) Relationships between serum adiponectin, leptin concentrations and bone mineral density, and bone biochemical markers in Chinese women. Clin Chim Acta 411:771–775
Gulhan I, Bilgili S, Gunaydin R, Gulhan S, Posaci C (2008) The effect of strontium ranelate on serum insulin like growth factor-1 and leptin levels in osteoporotic post-menopausal women: a prospective study. Arch Gynecol Obstet 278:437–441
Lajeunesse D, Pelletier JP, Martel-Pelletier J (2005) Osteoarthritis: a metabolic disease induced by local abnormal leptin activity? Curr Rheumatol Rep 7:79–81
Aspden RM, Scheven BA, Hutchison JD (2001) Osteoarthritis as a systemic disorder including stromal cell differentiation and lipid metabolism. Lancet 357:1118–1120
Gegout PP, Francin PJ, Mainard D, Presle N (2008) Adipokines in osteoarthritis: friends or foes of cartilage homeostasis? Joint Bone Spine 75:669–671
Cicuttini FM, Baker JR, Spector TD (1996) The association of obesity with osteoarthritis of the hand and knee in women: a twin study. J Rheumatol 23:1221–1226
Hu PF, Bao JP, Wu LD (2011) The emerging role of adipokines in osteoarthritis: a narrative review. Mol Biol Rep 38:873–878
Gualillo O (2007) Further evidence for leptin involvement in cartilage homeostases. Osteoarthritis Cartilage 15:857–860
Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P (2003) Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum 48:3118–3129
Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH (1988) Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys 267:416–425
Grimaud E, Heymann D, Redini F (2002) Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev 13:241–257
Simopoulou T, Malizos KN, Iliopoulos D, Stefanou N, Papatheodorou L, Ioannou M, Tsezou A (2007) Differential expression of leptin and leptin’s receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthritis Cartilage 15:872–883
Mutabaruka MS, Aoulad Aissa M, Delalandre A, Lavigne M, Lajeunesse D (2010) Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Res Ther 12:8
Anandacoomarasamy A, Giuffre BM, Leibman S, Caterson ID, Smith GS, Fransen M, Sambrook PN, March LM (2009) Delayed gadolinium-enhanced magnetic resonance imaging of cartilage: clinical associations in obese adults. J Rheumatol 36:1056–1062
Stannus OP, Jones G, Quinn SJ, Cicuttini FM, Dore D, Ding C (2010) The association between leptin, interleukin-6, and hip radiographic osteoarthritis in older people: a cross-sectional study. Arthritis Res Ther 12:19
Ku JH, Lee CK, Joo BS, An BM, Choi SH, Wang TH, Cho HL (2009) Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatol 28:1431–1435
Koskinen A, Vuolteenaho K, Nieminen R, Moilanen T, Moilanen E (2011) Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin Exp Rheumatol 29:57–64
Qin J, Shi D, Dai J, Zhu L, Tsezou A, Jiang Q (2010) Association of the leptin gene with knee osteoarthritis susceptibility in a Han Chinese population: a case–control study. J Hum Genet 55:704–706
Iliopoulos D, Malizos KN, Tsezou A (2007) Epigenetic regulation of leptin affects MMP-13 expression in osteoarthritic chondrocytes: possible molecular target for osteoarthritis therapeutic intervention. Ann Rheum Dis 66:1616–1621
Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Paivarinta U, Moilanen T, Moilanen E (2009) Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage—mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators Inflamm 345838:13
Brennan FM, McInnes IB (2008) Evidence that cytokines play a role in rheumatoid arthritis. J Clin Investig 118:3537–3545
Krysiak R, Handzlik-Orlik G, Okopien B (2012) The role of adipokines in connective tissue diseases. Eur J Nutr 51:513–528
Gomez R, Conde J, Scotece M, Gomez-Reino JJ, Lago F, Gualillo O (2011) What’s new in our understanding of the role of adipokines in rheumatic diseases? Nat Rev Rheumatol 7:528–536
Matarese G, Procaccini C, De Rosa V, Horvath TL, La Cava A (2010) Regulatory T cells in obesity: the leptin connection. Trends Mol Med 16:247–256
Notley CA, Ehrenstein MR (2010) The yin and yang of regulatory T cells and inflammation in RA. Nat Rev Rheumatol 6:572–577
Bokarewa M, Bokarew D, Hultgren O, Tarkowski A (2003) Leptin consumption in the inflamed joints of patients with rheumatoid arthritis. Ann Rheum Dis 62:952–956
Gunaydin R, Kaya T, Atay A, Olmez N, Hur A, Koseoglu M (2006) Serum leptin levels in rheumatoid arthritis and relationship with disease activity. South Med J 99:1078–1083
Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O (2006) Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis 65:1198–1201
Ibrahim SM, Hamdy MS, Amer N (2008) Plasma and synovial fluid adipocytokines in patients with rheumatoid arthritis and osteoarthritis. Egypt J Immunol 15:159–170
Derdemezis CS, Filippatos TD, Voulgari PV, Tselepis AD, Drosos AA, Kiortsis DN (2009) Effects of a 6-month infliximab treatment on plasma levels of leptin and adiponectin in patients with rheumatoid arthritis. Fundam Clin Pharmacol 23:595–600
Rho YH, Solus J, Sokka T, Oeser A, Chung CP, Gebretsadik T, Shintani A, Pincus T, Stein CM (2009) Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum 60:1906–1914
Seven A, Guzel S, Aslan M, Hamuryudan V (2009) Serum and synovial fluid leptin levels and markers of inflammation in rheumatoid arthritis. Rheumatol Int 29:743–747
Olama SM, Senna MK, Elarman M (2012) Synovial/serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatol Int 32:683–690
Lee SW, Park MC, Park YB, Lee SK (2007) Measurement of the serum leptin level could assist disease activity monitoring in rheumatoid arthritis. Rheumatol Int 27:537–540
Targonska-Stepniak B, Majdan M, Dryglewska M (2008) Leptin serum levels in rheumatoid arthritis patients: relation to disease duration and activity. Rheumatol Int 28:585–591
Popa C, Netea MG, Radstake TR, van Riel PL, Barrera P, van der Meer JW (2005) Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann Rheum Dis 64:1195–1198
Anders HJ, Rihl M, Heufelder A, Loch O, Schattenkirchner M (1999) Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metab Clin Exp 48:745–748
Nishiya K, Nishiyama M, Chang A, Shinto A, Hashimoto K (2002) Serum leptin levels in patients with rheumatoid arthritis are correlated with body mass index. Rinsho Byori 50:524–527
Hizmetli S, Kisa M, Gokalp N, Bakici MZ (2007) Are plasma and synovial fluid leptin levels correlated with disease activity in rheumatoid arthritis? Rheumatol Int 27:335–338
Popa C, Netea MG, de Graaf J, van den Hoogen FH, Radstake TR, Toenhake-Dijkstra H, van Riel PL, van der Meer JW, Stalenhoef AF, Barrera P (2009) Circulating leptin and adiponectin concentrations during tumor necrosis factor blockade in patients with active rheumatoid arthritis. J Rheumatol 36:724–730
Garcia-Bermudez M, Gonzalez-Juanatey C, Rodriguez-Rodriguez L, Vazquez-Rodriguez TR, Miranda-Filloy JA, Fernandez-Gutierrez B, Llorca J, Martin J, Gonzalez-Gay MA (2011) Lack of association between LEP rs2167270 (19 G>A) polymorphism and disease susceptibility and cardiovascular disease in patients with rheumatoid arthritis. Clin Exp Rheumatol 29:293–298
Harle P, Sarzi-Puttini P, Cutolo M, Straub RH (2006) No change of serum levels of leptin and adiponectin during anti-tumour necrosis factor antibody treatment with adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis 65:970–971
Gonzalez-Gay MA, Garcia-Unzueta MT, Berja A, Gonzalez-Juanatey C, Miranda-Filloy JA, Vazquez-Rodriguez TR, de Matias JM, Martin J, Dessein PH, Llorca J (2009) Anti-TNF-alpha therapy does not modulate leptin in patients with severe rheumatoid arthritis. Clin Exp Rheumatol 27:222–228
de Punder YM, Fransen J, Kievit W, Houtman PM, Visser H, van de Laar MA, van Riel PL (2012) The prevalence of clinical remission in RA patients treated with anti-TNF: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Rheumatology (Oxford, England) 51:1610–1617
Schett G, Kiechl S, Bonora E, Redlich K, Woloszczuk W, Oberhollenzer F, Jocher J, Dorizzi R, Muggeo M, Smolen J, Willeit J (2004) Serum leptin level and the risk of nontraumatic fracture. Am J Med 117:952–956
Barbour KE, Zmuda JM, Boudreau R, Strotmeyer ES, Horwitz MJ, Evans RW, Kanaya AM, Harris TB, Bauer DC, Cauley JA (2011) Adipokines and the risk of fracture in older adults. J Bone Miner Res 26:1568–1576
Wang L, Tang X, Zhang H, Yuan J, Ding H, Wei Y (2011) Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture. J Spinal Cord Med 34:501–509
Wang L, Yuan JS, Zhang HX, Ding H, Tang XG, Wei YZ (2011) Effect of leptin on bone metabolism in rat model of traumatic brain injury and femoral fracture. Chin J Traumatol 14:7–13
Park MC, Lee SW, Choi ST, Park YB, Lee SK (2007) Serum leptin levels correlate with interleukin-6 levels and disease activity in patients with ankylosing spondylitis. Scand J Rheumatol 36:101–106
Park MC, Chung SJ, Park YB, Lee SK (2009) Pro-inflammatory effect of leptin on peripheral blood mononuclear cells of patients with ankylosing spondylitis. Joint Bone Spine 76:170–175
Sari I, Demir T, Kozaci LD, Akar S, Kavak T, Birlik M, Onen F, Akkoc N (2007) Body composition, insulin, and leptin levels in patients with ankylosing spondylitis. Clin Rheumatol 26:1427–1432
Kim KJ, Kim JY, Park SJ, Yoon H, Yoon CH, Kim WU, Cho CS (2012) Serum leptin levels are associated with the presence of syndesmophytes in male patients with ankylosing spondylitis. Clin Rheumatol 31:1231–1238
Kushlinskii NE, Solov’ev YN, Babkina IV, Abbasova SG, Kostanyan IA, Lipkin VM, Trapeznikov NN (2000) Leptin and apoptosis inhibitor soluble Fas antigen in the serum of patients with osteosarcoma and neuroectodermal bone tumors. Bull Exp Biol Med 129:496–498
Yang SN, Chen HT, Tsou HK, Huang CY, Yang WH, Su CM, Fong YC, Tseng WP, Tang CH (2009) Leptin enhances cell migration in human chondrosarcoma cells through OBRl leptin receptor. Carcinogenesis 30:566–574
Wu YP, Chen WS, Xu SJ, Zhang N (2010) Osteoporosis as a potential contributor to the bone metastases. Med Hypotheses 75:514–516
Porter RW (2001) The pathogenesis of idiopathic scoliosis: uncoupled neuro-osseous growth? Eur Spine J 10:473–481
Burwell RG, Dangerfield PH, Moulton A, Anderson SI (2008) Etiologic theories of idiopathic scoliosis: autonomic nervous system and the leptin-sympathetic nervous system concept for the pathogenesis of adolescent idiopathic scoliosis. Stud Health Technol Inform 140:197–207
Qiu Y, Sun X, Qiu X, Li W, Zhu Z, Zhu F, Wang B, Yu Y, Qian B (2007) Decreased circulating leptin level and its association with body and bone mass in girls with adolescent idiopathic scoliosis. Spine 32:2703–2710
Liu Z, Tam EM, Sun GQ, Lam TP, Zhu ZZ, Sun X, Lee KM, Ng TB, Qiu Y, Cheng JC, Yeung HY (2012) Abnormal leptin bioavailability in adolescent idiopathic scoliosis: an important new finding. Spine 37:599–604
Tsuyama N (1984) Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res 184:71–84
Ikeda Y, Nakajima A, Aiba A, Koda M, Okawa A, Takahashi K, Yamazaki M (2011) Association between serum leptin and bone metabolic markers, and the development of heterotopic ossification of the spinal ligament in female patients with ossification of the posterior longitudinal ligament. Eur Spine J 20:1450–1458
Conflict of interest
All of the authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Chen, X.X., Yang, T. Roles of leptin in bone metabolism and bone diseases. J Bone Miner Metab 33, 474–485 (2015). https://doi.org/10.1007/s00774-014-0569-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-014-0569-7